MOJ
eISSN: 2574-9773


Short Communication Volume 1 Issue 5
11 Instituto de Macromoleculas, Universidade Federal do Rio de Janeiro, Brazil
2Universidade Veiga de Almeida, Centro de Sa
3Faculdade de Odontologia de Piracicaba, Departamento de Odontologia Restauradora, Universidade Estadual de Campinas, Brazil
Correspondence: Fernando G de Souza, Professor, Universidade Federal do Rio de Janeiro, Cidade Universitaria. Av. Horacio Macedo, 2.030. Centro de Tecnologia-Predio do Bloco J CEP 21941-598-Rio de Janeiro-RJ-Brasil, Tel 00 55 21 3938 7766
Received: October 28, 2017 | Published: November 29, 2017
Citation: Ricardo N, Souza FGD, Prado MD, et al. Gelatin as a chlorhexidine digluconate immobilizing agent. MOJ Poly Sci. 2017;1(5):183-186. DOI: 10.15406/mojps.2017.01.00029
Periodontal disease is an infectious-inflammatory disease that affects the supporting tissues of the teeth.This process results in inflammation within the supporting tissues of the teeth, progressive loss of attachment and bone loss and is characterized by periodontal pocket formation and/or recession of the gingiva. Chlorhexidine is a chemical agent widely used in dentistry, in the fight against periodontal disease and a wide range of infections, mainly thanks to its potent antimicrobial activity. Despite the excellent properties, chlorhexidine is an extremely hydrophilic drug, usually obtained in the aqueous solution form, making it hard to be incorporated into polymer controlled release systems. Successive lyophilization processes were inefficient for water removal. This work aimed to use gelatin as a chlorhexidine immobilizing agent. This procedure allowed the obtaining of a powder, which will be used as a drug controlled release film. The incorporation of chlorhexidine in a hydrophilic polymer matrix is already a well-established procedure. However, aiming the future preparation of a slower release device, we proposed the incorporation of the drug into a hydrophobic polymer matrix. To achieve this next purpose, it was necessary the immobilization of chlorhexidine in gelatin, which is reported here. More specifically, three gelatin and chlorhexidine systems were prepared. The characterizations were performed to understand the effect of different amounts of chlorhexidine on the obtaining of the system as a powder by lyophilization. Besides that, the capability of these systems to generate a microbiological response against two of the more significant microorganisms involved in the development of periodontal disease, Aggregatibacter actinomycetemcomitans (A.a), and Porphyromonas gingivalis (P.g), were evaluated as well. The results showed that the incorporation was carried out successfully. Also, the aspect of the obtained powder has a direct relation to the concentration of chlorhexidine. Regarding the microbiological test, the results show that all systems are capable of producing an efficient inhibition halo.
Keywords: controlled release of drugs, gelatin, chlorhexidine, periodontal disease
CRS, controlled drug release system; FTIR, fourier-transform infrared spectroscopy; TGA, thermogravimetric analysis; CHX, chlorhexidine G; DSC, differential scanning calorimeter; A.a, aggregatibacter actinomycetemcomitans; g, porphyromonas gingivalis
Periodontal diseases are a diversified group of clinical entities, described as pathologies of a multifactorial nature, in which the induction of an inflammatory process that destroys the fixation apparatus, loss of the alveolar bone and, if untreated, in the loss of the dental element. Among the wide variety of drugs used in the treatment of periodontal disease, chlorhexidine demonstrated to be a highly active chemical agent for microbial control. Chlorhexidine possesses the molecular formula C22H30Cl2N10, a molar mass equal to 505.4 g/mol, the density of 1.06 g/cm3 and a boiling point of 134°C.1 The antimicrobial effect of chlorhexidine is due to the attraction and adsorption of the chlorhexidine cationic molecules on the surface of the microorganism’s cells.2 This interaction promotes the alteration of the cell membrane permeability, resulting in the loss of intracellular components and the osmotic imbalance of the cell.3,4 Its sizeable microbial spectrum, persistence, and low toxicity,5,6 promoted the use of this substance in several areas related to the health. Also, the most widely used form of chlorhexidine is the 0.12% chlorhexidine digluconate solution.7 Despite presenting excellent antimicrobial activity, the effectiveness of conventional treatment of oral diseases is often limited due to reduced retention of formulations applied in the oral cavity. The salivary flow, the swallowing reflex, chewing and speaking may affect the dilution or displacement of a pharmaceutical formulation and can lead to a rapid decline in the concentration of the active ingredients to sub-therapeutic levels. Thus, there is a need for the development of drug delivery systems that could confer a better bio-availability of active components and a more significant retention of this active substance in the place where it should act while allowing a reduction in the frequency of administration and dosing.8 By definition, the Controlled Drug Release System (CRS) is an administration system designed to prolong the release time of the drug in the body, maintain its plasma concentration and control the temporal and spatial location of the molecules in vivo, by applying biological and chemical principles. Apart from this concept, there are already existing systems of local release of antibiotics or antiseptic solutions that are being used to restrict the action of the drug to the patient site and to minimize the systemic effects. The PerioChip® is a classic example of this type of apparatus. This device contains 2.5 mg of chlorhexidine gelatinous matrix. The literature showed that one of the major problems with this device is the initial burst of the unbound chlorhexidine, which is released in the first 48 hours, during what more than 40% of the chlorhexidine is released.9
The development of a device able to quickly reach the minimum CHX concentration besides keep as long as possible the drug release is the primary goal for better treatments. In this context, the use of polyesters deserves special mention because they have many applications in biomedicine due to their slow degradation, high permeability to many drugs and low toxicity. Thus, the failure to encapsulate chlorhexidine10,11 to the polymer matrix of interest via emulsion due to incompatibility between both components is a stimulus to the development of a powder formulation so that the drug could be incorporated into the polymer matrix and thus, a more efficient controlled release system could be developed for use in the oral cavity. To achieve this goal, gelatin, a natural polymer produced from collagen hydrolysates or the polysaccharide fraction of seaweed was used as a Chlorhexidine immobilizer.12-16 Differently from other studies, which were focused on the use gelatin as the primary polymer matrix, the present study aims to obtain a powder of chlorhexidine in a fast and cheap way. Aiming this, gelatin was used as an immobilizing agent. Thus, the obtained gelatine-chlorhexidine powder would easily be incorporated into another polymer matrix by a simple melt mixing method, making easier the obtaining of a device for the even slower release of the drug and a prolonged local time of action.
Materials
Gelatin powder, from SYNTH and Chlorhexidine gluconate (CHX) 20% (w/v), from MIL FORMULAS, Brasil. All the reactants were used as received.
Methods
Gelatin-Chlorhexidine mixtures: The mixtures were prepared using the same amount of gelatin (10 g). To all systems, the final volume of the obtained solution was equal to 50 ml. Soon afterward the complete dissolution of the gelatin, the following volumes of chlorhexidine was added to each system: 15mL (3g of CHX), 25mL (5g of CHX) and 50mL (10g of CHX). These systems were named as 3G, 5G, and 10G, respectively. The amounts of CHX in the systems 3G, 5G and 10G were equal to 23,1%, 33,3%, and 50%, respectively. The mixtures were poured into beakers for 2 min, and subsequently, the obtained materials were poured into 4 glass plates. These plates were placed in the refrigerator for 24 hours. After 24 hours in the refrigerator, the Gelatin-Chlorhexidine mixtures were passed through a bath in liquid nitrogen and lyophilized to remove excess water in a Liotop L101 freeze dryer. The 3G (3g of CHX), 5G (5g of CHX), and 10G (10g of CHX) samples were lyophilized for 30 h, 24 h, 18 h, respectively. The different lyophilization times had the objective of removing the water until reaching constant mass. Once the excess water was removed, the samples were ground with the aid of an IKA A11 crusher and then sieved in a 100 μm aperture particle size sieve until a fine powder was obtained for each system.
The microbiological test was performed to establish a comparison with the future polymeric devices developed. The test is based on the formation of an inhibition zone demonstrating that antimicrobial activity of the samples of gelatin-chlorhexidine in a test of agar diffusion front of the following microorganisms: Aggregatibacter actynomicetemcomitans (A.a) and Porphyromonas gingivalis (P.g). The microorganisms were selected taking into consideration that A.a and P.g are among the leading etiological agents of periodontal disease. Pure gelatin and 0.12% Perioxidine gel (Lacer·) were used as the control group.
Aiming to standardize all the parameters, the obtained materials were subjected to different lyophilization times to eliminate the excess of water. The difference in these lyophilization times is related to the decrease of the intermolecular forces. Thus, the 10G group needed the smaller lyophilization time. A linear correlation test was performed to prove the relationship between the CHX concentration and the time of lyophilization, and the obtained value was equal to-0.99 which indicates that there is a high correlation between the measured parameters. This result is shown in Table 1.
Sample |
CHX (%) |
Lyophilization Time |
3G |
23.1 |
30 |
5G |
33.3 |
24 |
10G |
50 |
18 |
Correlation |
-0.99 |
|
Table 1 Relationship between the amount of distilled water and the lyophilization time of each sample
Figure 1 shows the TGA of the samples. Obtained data allowed inferring that the CHX solution lost about 79% of its mass up to 117°C, proving that the drug content indicated by the manufacturer is correct, being around 20%. Besides that, the drug remains stable up to 160°C. Soon afterward, the CHX presents two degradation events. The first finished at 244°C, and the second at 497°C. The gelatin lost H2O up to 145°C, leaving a 40% residue of gelatin which starts to degrade at 281°C. In turn, the systems contained CHX in gelatin presented a common thermal behavior: as samples were thermally stable up to 130°C. Then, they suffered 2 degradation steps. 3G, 5G, and 10G samples presented their first maximum degradation rate at 202°C, 206°C, and 205°C, respectively. The same samples presented their second maximum degradation rate at 348°C, 346°C, and 346°C, respectively. Therefore, the temperature used in the preparation of the samples did not affect the CHX, and the mixing process can be considered secure to the drug.
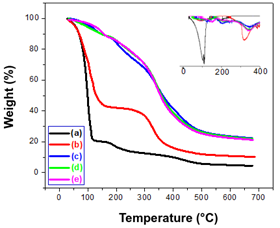
Figure 2 shows the DSC of the samples. CHX presents an endothermic event around 106°C. This event is attributed to the evaporation of the water present. There is another endothermic event smaller, which took place around 186°C. This is due to the fusion of chlorhexidine.17 Pure gelatin has an endothermic peak ranging from 46°C to 160°C. This event occurs due to the loss of water and it is longer than the CHX one due to hydrogen bonds between the gelatin and the water. Systems containing the lyophilized mixture of CHX and gelatin were not influenced by the presence of water since they were analyzed immediately after lyophilization. These systems presented endothermic peaks between 155°C and 180°C, which are related to the melting of CHX.
The Fourier Transform Infrared Spectroscopy technique was used as a characterization method to prove the incorporation of the drug into the gelatin. Figure 3 shows a comparison between pure gelatin spectra and samples containing CHX. All the samples containing the drug presented the N-H characteristic band of the CHX amine at 1640 cm-1. This band was used as a reference for the present amount of CHX. As one can see in the inset of Figure 3, as higher the amount of CHX, as intense the transmittance of the CHX amine peak. The FTIR band centered at 1640 cm-1 was used to correlate the chlorhexidine concentration present in these samples with the transmittance. The obtained results allowed inferring that the higher the chlorhexidine concentration, the greater the transmittance of the 1640 cm-1. The corresponding coefficient of determination (R2) was equal to 0.9588. Therefore, the increasing amount of CHX in the materials was proved.
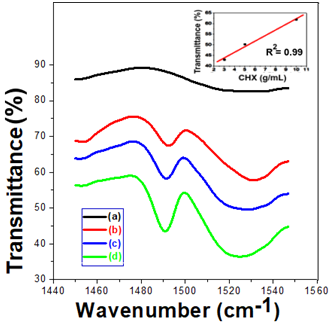
Table 1 shows the average results obtained using triplicates of the microbiological test for A.a, and P.g. The Figure 4 & Figure 5 showed the inhibition halo of the tested groups, from which data of Table 2 was obtained. The results allowed inferring that each one of the developed gelatine-chlorhexidine systems produced more magnificent inhibition halos than the commercial formulation. Moreover, it is noted that the systems produce higher antimicrobial activity against P. g. Therefore, the obtained results proved the encapsulation of the CHX in the gelatin, which can be the basis for the preparation of cheaper drug delivery systems for the treatment of several dentistry diseases.
Samples |
A.a (mm) |
P.g (mm) |
3G |
21.7±4.7 |
38.1±0.2 |
5G |
20.3±1.1 |
37.3±0.2 |
10G |
21.1±0.7 |
36.4±0.9 |
Pure Gelatin |
0.0±0.0 |
0.0±0.0 |
Perioxidine |
15.0±0.0 |
32.0±0.0 |
Table 2 Inhibition halo test performed in triplicate for the A.a and P.g. microorganisms
Gelatin can be used as the hydrophilic drug immobilizing agent. Besides, unlike other controlled release devices for CHX based on polymer systems, the material presented here were prepared without the use of organic solvents, which would be a way of making the release devices cheaper and more readily available for the population.
The authors wish to acknowledge development institutions that have made this work possible: CNPq, CAPES, FINEP e a FAPERJ. Coleção de Microrganismos de Referência em Vigilância Sanitária – CMRVS Fundação Oswaldo Cruz - FIOCRUZ-Instituto Nacional de Controle de Qualidade em Saúde - INCQS-Lab. de Microrganismos de Referência
The author wish to state that there is no conflict of interest.

©2017 Ricardo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.