MOJ
eISSN: 2574-9773


Mini Review Volume 1 Issue 1
Bhabha Atomic Research Centre, India
Correspondence: Subhendu Ray Chowdhury, Isotope and Radiation Application Division, Bhabha Atomic Research Centre, Trombay, Mumbai-400 085, India
Received: February 21, 2017 | Published: March 17, 2017
Citation: Jha A, Sarma KSS, Chowdhury SR. Advancement of oil/water separating materials: merits and demerits in real-time applications. MOJ Poly Sci. 2017;1(1):11-16. DOI: 10.15406/mojps.2017.01.00003
With the increase of offshore drilling of oil, production and transportation the chances of oil spillage has enormously increased. Oil spillages have a catastrophic impact on our aquatic environment and ecosystem. In the last few years development of special wettable materials for oil-water separation has received tremendous research and industrial interest. Materials with selective wettability, that is superhydrophobic and superoleophilic, or superhydrophilic and superoleophobic, can be used to remove only one phase from the oil/water mixture. Moreover, the effect of the surface chemistry and surface architecture can further promote the superwetting behaviour and improves separation efficiency. In this review, recently developed materials for oil/water separation are summarized and discussed. These materials have been categorized based on their oil/water separating mechanisms that is filtration or absorption. Representative studies are highlighted, with emphasis on the materials wetting properties that is superhydrophobic/superoleophilic or superoleophobic/superhydrophilic nature, innovative aspects and their applications. The materials with selective wettability can be used for the treatment of oil spills and industrial oily wastewater treatment on an industrial scale. The challenges and future research directions in this emerging and promising research field are briefly described.
Keywords: oil-water separation, super wetting property, superhydrophobic, superoleophilic
In the last few years, there are hundreds of large oil spill incidents throughout the world, among them Montara oil spill (Australia), Deep water horizon (Gulf of Mexico), Xingang Port oil spill are the most alarming. In India also Mumbai oil spillage 2010 and Chennai oil spillage 2017 leave severe environmental consequences. Oil spillage is one of the most serious environmental and ecological challenges.1,2 Oil contamination from various industries is also causing considerable amount of water pollution. The marine atmosphere and sea water are the most affected due to these incidents, so there is an urgent need of development of material for collection and separation of oil and oily organic pollutant from water sources.
The techniques which are being used to clean up such leakage or spillage are mainly skimming, addition of chemical dispersant, use of filtrating medium and various sorbents. Skimming is the most common method for this purpose, but it is very time consuming, costly and inefficient. Second most widely used method is use of oil dispersant, which separates oil into droplets, but this method adds additional toxic chemical into sea and requires additional separating techniques.3,4 Use of filtrating materials and sorbents are the most effective and convenient way to the clean and recollects oil from oil spillage.5,6 In the last five years, there is an exponential growth in the research related to materials for oil-water separation. Purposefully constructed and specially designed super wetting surface are found to be helpful for selectively separating oil and water. In particular, special wettable materials with distinct opposite affinities towards oil or water are believed to be the most promising materials for selective oil/water separation.7–9 The subject to be covered in the following sections are various separation techniques, materials and scientific basis of those.
Specifically, two kinds of special wettable materials are suitable for oil/water separation, i.e., hydrophobic-oleophilic materials, and hydrophilic-oleophobic materials. Superhydrophobic-superoleophilic materials are actually oil removing materials from the bulk. This particular type of wetting property of material will repel the water phase and absorb oil, thus separating oils from an oil and water mixture. Superhydrophilic-superoleophobic materials are water removing material from the oil-water mixture with the reverse selectivity to the oil absorbing materials. Various metallic mesh material, fabric material, carbon based material, powder material, sponge or foam material are reported till date which can be used as oil water separation,10–19 but one major drawback of these fabric and mesh materials is that, they cannot be used directly in the sea, oily water has to be collected and then it has to be filtered. These are difficult to scale-up in large scale.20 Most effective sorbent reported are the sponge and foam based material because of their easy availability, low cost, very low weight to volume ratio and extremely high porosity they can absorb oil up to hundred times of their weight, but they have less selectivity towards oil or water, they absorb both almost equally. Purposefully constructed and specially designed super wetting surface are found to be helpful for selectively separating oil and water. Drop coating, solution coating, fabrication by electro-spinning, plasma treatment, incorporation of nano particle into sponge material etc. various methodology have been used to prepare such sponges.21–23 But this methods require toxic chemicals, involve high cost, less scalability in large scale and low reusability. The main emphasis is given into the specialty of the materials, surface wet ability and merits and demerits. Finally, we provide our conclusions and outlook on the future of the work.
Filtration based separation: Depending upon the wettability, modified filtrating materials like metallic meshes, textiles/fabrics, polymeric membranes, allow only one particular phase to pass through. These materials are extensively studied in the last few years for oil-water separation.
Metallic mesh-based materials: Superhydrophobicity of metallic meshes has been studied over the past few years.11,24–26 To enhance the selective permeability to water and oil, filtering mesh with superhydrophobic and superoleophilic behaviour is prepared, resulting in oil/water separating properties. Feng et al.27 has reported the first super-hydrophobic and superoleophilic stainless steel mesh prepared via a spray and-dry method in 2004.27 The surface morphology of the mesh is rough and because of its superhydrophobicity and superoleophilicity, water droplet stays as a spherical bead on the mesh and oil (diesel) droplet passes through it (Figure 1). In addition to stainless steel mesh, copper mesh is one of the another frequently used substrates for oil/water separation.28–33 Wang et al.30 reported a superhydrophobic and superoleophilic copper mesh, This as prepared mesh could be used to separate a mixture of oil and water. Similarly, superhydrophilic and superoleophobic meshes can also be used for the same purpose. Preparation of these kinds of material which show both hydrophilicity and oleophobicity is difficult as surface energy of water is higher than oil.9 Yang et al.34 reported a polymer based superhydrophilic and superoleophobic coating material, prepared from hydrophilic poly (di-allyl di-methyl ammonium chloride) (PDDA) and oleophobic sodium perfluroocanoate (PFO) and SiO2 nanoparticles. It allows water to spread through the mesh and oil drop keeps minimum contact with the surface and stays as a spherical bead (Figure 2). Kota et al. reported hygro-responsive superhydrophilic and superoleophobic membrane material by dip-coating method.35 Most of the oil are of less density than water, so a filtrating material with superhydrophilicity and oleophobicity will be more useful and feasible for oil-water separation, if the oil-water mixture is a layered (immiscible) system. Mesh material with underwater superoleophobic superhydrophilic surface can be designed by appropriate surface energy and surface roughness.36,37 These materials are also applicable to oil-water separation. Xue et al.36 reported polyacrylamide-hydrogel coated mesh with underwater superoleophobicity. These type of materials overcome the disadvantage of fouling due to oil absorbance which was a major issue with superhydrophobic-oleophilic materials.

Figure 1 (A) Scanning electron microscopy (SEM) images of the stainless steel mesh coating; (B) high resolution view of (A). (C) The shape of a water droplet on the resulting mesh film with a water contact angle (WCA) of 156.2ᵒ±2.8ᵒ; (D) the diesel oil spreads and penetrates the copper mesh film quickly within only 240 ms, indicating an oil contact angle (OCA) of 1.4ᵒ 27.
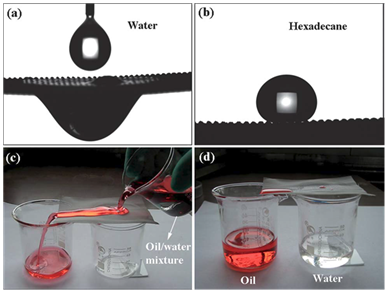
Figure 2 The PDDA–PFO/SiO2-coated mesh film shows special wettability, with both superhydrophilic and superoleophobic properties. (A) Water droplet spreading on and permeating through the mesh. (B) Shape of a hexadecane droplet on the mesh with a contact angle of 157ᵒ±2ᵒ. (C and D) Oil/water separation experiment was performed on the PDDA–PFO/SiO2-coated mesh 34.
Fabric/textile based materials: Apart from inorganic porous metal films, soft and flexible organic fabric materials have also been found as a good candidate for oil/water separation. Zhang et al.38 reported a superhydrophobic cotton textile with oil/water separating properties by dip coating method. Tang et al.39 and Shang et al.40 fabricated a superhydrophobic and superoleophilic nanofibrous membrane with oil/water separation properties using a combination of electrospun poly(m-phenyleneisophthalamide) (PMIA) nanofibers and a novel in situ polymerized fluorinated polybenzoxazine (F-PBZ) functional layer that incorporated SiO2 NPs. Zhang et al.41 proposed a superhydrophobic and superoleophilic polyester textile using a one-step growth of silicone nanofilaments onto the textile via chemical vapour deposition method. Zhang et al.42–44 reported couple of durable and robust superhydrophobic textiles with good mechanical, chemical and environmental stability. Oil-water mixture can be separated by using very simple experimental set up using these textile materials (Figure 3). Few materials have been reported which show responsive wettability. Xu et al.45 reported ammonia responsive superhydrophilic and superoleophobic polyester textile material. Xiong et al.46 reported a unique simple textile material for switchable wettability by using ZnO nanorod deposited polyethylene terepthalate, which displays underwater oleophobicity and under oil hydrophobicity. The developed fabrics, prepared using various physical and chemical routes, has been found to be useful for separating oil-water mixture. But the major drawback of these materials is that the contaminated water has to be collected first and then can be separated, we can not use these filtering materials directly in real sites.9
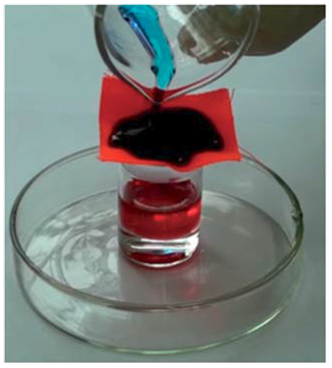
Figure 3 Simple experimental setup for oil/water separation using modified textile material. Oil is colored with Or range red and water is colored with Methyl blue 43.
Although various advanced filtration materials with special and tunable wetting properties have been developed for selective oil/water separation, they still have difficulties for the in-situ application. In the last couple of years, much research effort has been devoted to developing advanced absorbents with special wetting properties that can selectively separate oil-water mixture.
Particle and Powdered Absorbents: Powdered materials, such as activated carbons and zeolites, iron nano particles etc. have been studied by various research groups. Arbatan et al.47 reported a superhydrophobic and oleophilic calcium carbonate powder that can separate oil from an oil water mixture quickly.47 Hydrophobic polymer coated silica particles has been reported to be useful for petroleum hydrocarbon removal by Akhavan et al.48 Recently developed few metal organic frameworks (MOF) with special wettability are also found to be very promising in this regard.49–51 Yong et al.52 very recently reported an underwater superoleophobic pre wetted sand material for oil-water separation. However, these traditional powered absorbents very difficult to handle, especially during the re-collection process. Magnetic particles/powders were developed to overcome the recycling problem because particles/powders with magnetism can be easily collected using an external magnetic field.53 Though the materials are found to be useful in oil-water separation, its low absorption capacity and less stability in harsh environment hinder its wider acceptance.9
Sponge based materials: Sponge and foam based materials have gained considerable interest in this aspect because of its inherent properties like easy availability, low cost, porous, high absorbance capacity, with initial wettable properties. Usually, they can absorb various liquids (including water and oils or organics), which make them unrealistic for removing oils/organics from the water phase because of their poor selectivity. High selectivity has been introduced onto these materials by purposeful construction of befitting surface topography and modification by functional molecules with special wetting property. Different promising routes such as in situ growth,54 dip coating,55 polymerized octadecylsiloxane coating,56 carbon nanotube/poly(dimethylsiloxane) based coating,23 block copolymer (BCP) grafting,57 solution-immersion,58 sugar templating,59 vapour phase polymerization60 for developing special wettability of these materials have been followed by various research groups. Coating of various low surface energy molecule on the surface of sponge based materials are widely used means for attaining superhydrophobiciy and superoleophilicity.23,58,61–63 Shuai et al.64 reported polydimethylsiloxane –TiO2 coated superdydrophobic polyurethane (PU) sponge. Various carbon based materials like CNT, grapheme, etc have been applied as a hydrophobic coating /modifying agent.65–67 One disadvantage associated with most polymeric sponges is their instability. These materials cannot withstand the situation related to site applications, such as bending, twisting, pressing etc. Few groups have reported superhydrophobic-superoleophilic melamine based sponge material with extensive temperature and fire resistance.68,69 Our group has recently introduced a rapid, single step, scalable, economic and sustainable route to incorporate super selectivity towards oily liquid to the sponge upon modification via gamma radiation assisted grafting of a low surface energy molecule (dodecyl 2-methacrylate).70 In a oil-water mixture it absorbs only oil and cleans the water, the absorbed oil can be easily recollected by squeezing (Figure 4 & Figure 5). This is the first material which can separate oil/water from both layered and emulsion oil/water mixtures. Wang et al.71 reported a cellulose sponge with robust superhydrophilicity and under-water superoleophobicity for highly effective oil/water separation. Smart surfaces are able to switch between superoleophilicity to superoleophobicity in presence of water on any other stimuli like pH or temperature. Zhang et al.57 used a pH responsive poly (2-vinylpyridine) (PVP) and oleophilic hydrophobic polydimethylsiloxane (PDMS) as coating materials onto sponge surfaces for pH responsive wettability.57 Recently few innovative strategies has been developed for continuous separation of oil-water which out ways the absorbance capacity of some materials.72 Ge et al.73 designed a pump with the sponge for absorbent-pumping system (Figure 6).

Figure 4 (A-H) Images of contact angles (A and B, E and F) and beading (C and D, G and H) of liquid water (A, C, E and G) and oil (B, D, F and H) droplets on both the pure-PU sponge (E-H) and DMA-grafted PU sponge (A-D) 70.

Figure 5 (A-D) Oil/water separation and recollection of oil from the modified sponge by mechanical squeezing 70.
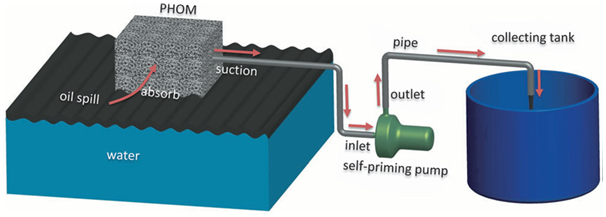
Figure 6 Diagrammatic representation of sponge based absorbent-pumping system for continuous oil-water separation. The oil on the water surface is selectively absorbed by the sorbent and flows along the pipe into the collecting vessel until all oil is consumed 73.
In this article, we have reviewed recently developed various novel materials based on both filtration and absorption methods. The required wetting properties include hydrophobicity and oleophilicity, hydrophilicity and oleophobicity, superhydrophilicity and underwater superoleophobicity, and responsive/ switchable wettability. Although oil/water separation with special wettable materials is a rapidly growing and promising research field, there are still some challenges to overcome. There is still a lack of understanding of the basic mechanisms of interactions between oil and the surface of the materials used to clean up the oil. Fundamental theories and models are required in order to guide further development of novel materials. Among the reported works, for many of the cases the synthesis methods cannot be performed on large-scale (such as the in situ growth method and hydrothermal method) and thus the mass-production techniques of oil/water separation materials for the large-area oil spills need to be developed. Very few reported works have addressed the separation of oil-water emulsions. Effective and high-throughput separation of a wide range of oil/water emulsions with a range of droplet sizes from the micrometer to the nanometer range is an important issue and need more research interest. In most of the cases the studies have been performed using low-viscous oil, however, research on the separation of high-viscous oil-and-water mixtures are rare. Thus future research is required to be carried out to overcome the aforementioned challenges. We believe this mini-review could generate more research interest in this blooming area.
None.
The author declares no conflict of interest.

©2017 Jha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.