MOJ
eISSN: 2574-9773


Case Report Volume 1 Issue 5
Department of Chemical Engineering, Ryerson University, Canada
Correspondence: Yaser Dahman, Department of Chemical Engineering, Ryerson University, 350 Victoria St., Toronto, Ontario M5B 2K3, Canada, Tel 416-979-5000
Received: October 23, 2017 | Published: November 27, 2017
Citation: Ghalia MA, Dahman Y. Bacterial cellulose nanofiber reinforced poly (lactic acid) nanocomposite. MOJ Poly Sci. 2017;1(5):174-176. DOI: 10.15406/mojps.2017.01.00027
The objective of the present work is to investigate the impact of the modified BC nanofibers orientation in poly (lactic acid) (PLA)/polyethylene glycol (PEG) blends (porous and nonporous) on the mechanical properties and thermal properties. A new class of PLA/PEG biocomposites containing BC nanofibers was successfully fabricated using solution casting technique followed by silane coupling agent of BC grafting into PLA/PEG chains. The mechanical properties were investigated with and without developing the porosity for PLA/PEG. The results revealed that incorporating BC nanofibers into PLA/PEG enhanced mechanical properties, but this improvement set up at BC nanofiber loading (5wt%). The tensile strength increased from 13MPa for PLA/PEG to 18MPa after the addition of 5wt% BC. The young’s modulus was significantly increased upon increased BC content. Differential Scanning Calorimetric (DSC) results revealed that the BC 5% nanofiber improved glass transition temperature (Tg) to 57°C, melting temperature (Tm) to 171°C, and crystallinity (χ%) to 43% of PLA/PEG reinforced-BC-5%. These results are of significant interest to further expand the use of PLA in biomedical applications.
Keywords: poly (lactic acid), bacterial cellulose nanofibers, polyethylene glycol, nanocomposites
PLA, poly(lactic acid); PEG, polyethylene glycol; DSC, differential scanning calorimetric; Tm, melting temperature; Tg, glass transition temperature; VST, vicat soft temperature; BC, biocellulose
Recent developments in the synthetic degradable polymers have been the subject of significant interest for macromolecular science to both of an environmental and biomedical perspective with dedication towards applications in tissue engineering and drug delivery. The best candidate for degradable polymer is poly (lactic acid) (PLA), which is described as aliphatic polyester.1 It is produced from renewable resources of agriculture via combined fermentation and polymerization processes. Considering that PLA has several remarkable properties, such as relatively high modulus and hardness at ambient temperature, it is a candidate as a replacement for traditional petroleum based polymers. In contrast, the PLA application is still restricted because of the low glass transition temperatures (Tg), vicat soft temperature (VST), and frailty.2,3 The blending of binary degradable polymers and copolymerization can enhance the limitation of mechanical properties.4 However, immiscibility and non-compatibility are considerable barriers in developing PLA with superior properties.5
Biocellulose (BC) nanofibers are potentially considered as one of the utilizing natural resources to employing into the degradable polymer. Lately, several scientists are attracted to the BC nanofibers, due to the superiority in biocompatibility and mechanical properties. BC can be prepared from fermentation process throughout a specific type of bacterial strains. The unique structure of BC is formed from the pure form of cellulose nanofibers, which contains a high degree of crystallinity associated with a high degree of polymerization.6 These exceptional properties also possess potential forerunner for development technologies in the various significant area, such as tissue engineering, sustainable polymeric biomaterials, and green nanocomposites.7 BC nanofiber reinforced into PLA nanocomposite has gained much consideration because of their low density, inexpensive, non-abrasiveness; very low toxicity and degradable properties. Several reports6-8 revealed that the mechanical and thermal properties of degradable polymers are enhanced when using an appropriate Compatibilizer with the cellulose nanofibers. The non-uniform dispersion can cause inferior tensile strength and high-water absorption. Hence, the suitable interface Compatibilizer agent between the PLA matrices and BC nanofibers would lead to improving BC distribution. Meanwhile, BC contents reduced surface polarity when interacting with PLA and improved reinforced efficiency.9,10
In recent years, surface modification of (BC) by grafting coupling agent into PLA matrices has demonstrated a special interest in reducing the number of hydroxyl groups and enhance the surface hydrophobicity of BC nanofiber.11 Silane chains can enhance interaction between PLA matrices and BC nanofibers.12 Moreover, the presence of polyethylene glycol (PEG) in PLA blends lead to improving hydrophilicity and accelerates its degradation rate. Various silanes are potentially considered as effective agents in enhancing the interface between PLA/PEG blends and BC nanofibers. This involves the alkoxysilanes creating bonds with BC hydroxyl function groups. Additionally, the organ functional group is spontaneously reacting with PLA through covalent bonding 13.
Preparation of the PLA/PEG copolymer-BC nanocomposites
The PLA/PEG was prepared in a reaction vessel equipped with an overhead stirring shaft. The copolymer and BC were heated at 60°C for 2 h until the relative vapor content was less than 1.0 wt%. Afterward, the PLA/PEG heated for about half an hour at 110°C, ensuring all PLA/PEG convert to the high viscous solution, and later on the BC suspension solution was mixed with a presence of dicumyl peroxide for 15 min at 170°C and rotation speed 60 rpm. The peroxide produced a free radical on the BC surface to further reacted with hydrogen group of copolymer. The BC nanofiber amounts were 1 wt%, 2.5wt%, 5 wt%, and 10 wt%. The BC treated coupling agent was grafted onto the PLA/PEG side chains and therefore form BC-reinforced PLA/PEG as illustrated in the reaction (Figure 1). All the samples were poured using tensile prototype molding (2 mm thick) at 110°C and subsequently kept for 24 h at ambient temperature under vacuum to eliminate the solvent and allow the copolymer chains to reconstruct.
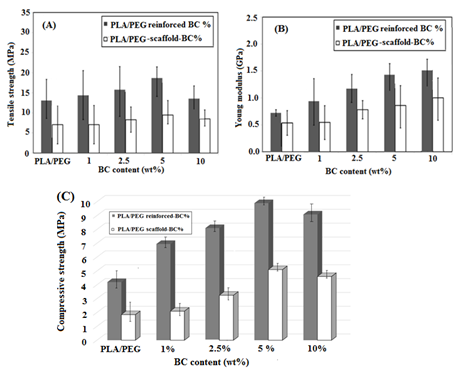
The addition of BC content in PLA/PEG demonstrated a different trend in tensile strength properties. It is clearly seen from Figure 2(A) that the tensile strength of the PLA/PEG was enhanced as a function of increased BC loading up to 5 wt% and a drastic decrease with the addition of BC-10 wt%. In the meantime, the young’s modulus of PLA/PEG reinforced-BC% and PLA/PEG-scaffold-BC% increased with an addition of BC content as represented in Figure 2(B). The compressive strength obtained for PLA/BC-5 wt% was 9.8 MPa, which significantly dropped after BC-10wt% to 8.8 MPa (Figure 2(C)).
It is noted that both the Tg and Tm in Table 1 are slightly increased relative with the increased BC nanofibers. PLA/PEG contains Tg, Tc, and Tm of 53, 121, and 168°C respectively. The Tg of PLA/PEG scaffold without BC stayed at approximately 51°C, which indicates that weak interfacial bonds among the copolymers. An increase in the degree of crystallinity 43% of PLA/PEG blends with an addition of BC-5% attributed to the fact that the PEG acts as plasticizers in the PLA matrices. As well as, the addition of BC% constricting PLA/PEG chain movements. In addition, both the ∆Hc and Tc decrease with increased BC nanofiber. This indicates that BC is acting as a nucleating agent, and promoting crystallization in the PLA/PEG copolymer.12,13
Code |
Samples |
Tg(°C) |
Tc (°C) |
Tm (°C) |
ΔHc (J/g) |
ΔHm (J/g) |
χ% |
A |
PLA/PEG |
53 |
121 |
168 |
14.3 |
9.3 |
33 |
B |
PLA/PEG reinforced-BC 1% |
54 |
119 |
168 |
13.7 |
12.5 |
35 |
C |
PLA/PEG reinforced-BC 2.5% |
54 |
119 |
169 |
12.6 |
15.1 |
37 |
D |
PLA/PEG reinforced-BC 5% |
57 |
116 |
171 |
12.9 |
25.8 |
43 |
E |
PLA/PEG reinforced-BC 10% |
55 |
115 |
158 |
11.5 |
19.7 |
35 |
F |
PLA/PEG scaffold |
51 |
118 |
162 |
10.4 |
4.6 |
24 |
G |
PLA/PEG scaffold -BC 1% |
45 |
113 |
157 |
8.6 |
8.1 |
26 |
H |
PLA/PEG scaffold-BC 2.5% |
47 |
110 |
156 |
7.2 |
8.7 |
28 |
I |
PLA/PEG scaffold-BC 5% |
48 |
91 |
156 |
6.8 |
10.8 |
29 |
J |
PLA/PEG scaffold-BC 10% |
43 |
87 |
154 |
5.7 |
7.2 |
22 |
Table 1 Thermal stability of PLA/PEG with and without porous scaffolds incorporated with varying of (BC 1-10 wt%)
A new class of polylactic acid (PLA)/polyethylene glycol (PEG) copolymer reinforced with bacterial cellulose nanofibers (BC) was prepared using a solvent casting and particulate leaching methods. The results of mechanical and thermal properties demonstrated that the PLA/PEG synthesized is good candidate for developing scaffolds for three-dimensional porous structures, which can be implemented into various tissue engineering applications such as surgical sutures, and drug delivery devices.
None.
The author declares no conflict of interest.

©2017 Ghalia, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.