MOJ
eISSN: 2574-9773


Mini Review Volume 1 Issue 1
Departamento de S
Correspondence: Ricardo Acosta Ortiz, Centro de Investigacion en Quimica Aplicada, Blvd Enrique Reyna #140, Saltillo, Coahuila Mexico, Tel 52844 4389844
Received: March 30, 2017 | Published: April 17, 2017
Citation: Acosta Ortiz R. Recent advances in the anionic photocurable Epoxy/thiol-ene systems. MOJ Poly Sci. 2017;1(1):36-39. DOI: 10.15406/mojps.2017.01.00006
This review discusses the characteristics of the epoxy/thiol-ene systems such as high reactivity and the improvement of toughness of the obtained polyether-polythioether co-network. This review also examines the applications of this system in the preparation of glass-fiber reinforced epoxy resins (GFREC), shape memory materials (SMP), foamed epoxy polymers and self-healing polymers.
Keywords: epoxy resins, thiol-ene, toughness, foamed epoxy polymers, self-healing, shape memory, glass-fiber epoxy composites
Epoxy resins are highly utilized in the industrial sector due to several advantages including superior mechanical properties, chemical and heat resistance, low shrinkage, high adhesivity and low toxicity.1 However, one of the main drawbacks is the inherent fragility due to the high level of crosslinking in these materials. Because of this aspect, several methods to improve their toughness have been reported.2,3 Our research group focused on improving the toughness of photocurable epoxy resins by concurrently polymerizing them with a thiol-ene system.4,5 Thiol-ene photopolymerization has recently been classified as “click” chemistry because virtually any kind of thiol can react very rapidly under mild conditions, with any unsaturated compound, even in the presence of oxygen, to produce polythioethers.6 Recent investigations by our group have demostrated that simultaneous anionic polymerization of an epoxy resin and a thiol-ene system result in the formation of homogeneous polymeric materials with improved toughness.7 This result is due to the flexible polythioethers that originate during thiol-ene photopolymerization. The epoxy/thiol-ene system was composed of an epoxy resin such as the diglycidyl ether of bisphenol A (DGEBA), and the thiol-ene system, The thiol-ene system included a curing agent of the tertiary amine type, such as N1, N1, N6, N6 tetrallyl hexane-1,6-diamine (ALA4) and a radical photoinitiator like dimethoxyphenyl acetophenone (DMPA) (Figure 1). The formulations were subjected to a simultaneous dual photo-thermal cure at 85°C and a 40mW/cm2, while varying the molar concentration of the thiol-ene system in the epoxy resin. This epoxy/thiol-ene system was demostrated to be highly reactive achieving full conversion in less than 10 minutes.8 This reactivity can be described to the presence of several initiating species such as the tertiary amine, thiolate, and thioethers groups. They are all basic species that are able to react with the oxirane groups promoting their anionic ring opening polymerization (Figure 2). It can be seen that the tertiary amine attacks the epoxide groups which undergo Hoffman intramolecular re arrangement to produce vinyl alkoxide responsible for the initiation of the anionic ring opening polymerization of the oxirane groups. The tertiary amines are also able to undergo acid-base reaction with the thiol groups to produce thiolates. These species are basic enough to anionically attack the epoxide groups. At the same time the thiol groups can react with the double bonds of the curing agent to produce polythioethers. When the concentration of the polythioethers is significant, they can also attack the residual epoxy groups to achieve full conversion of the epoxide groups depending on curing conditions.
We have taken advantage of the properties of these epoxy/thiol-ene systems to prepare glass-fiber reinforced epoxy composites (GFREC), shape memory materials (SMP), foamed epoxy resins and self healing epoxy systems.
Glass-Fiber Reinforced Epoxy Composites (GFREC)
Regarding the glass-fiber composites, our photocurable glass-fiber/epoxy formulations using the thiol-ene system as initiator were completely cured in 400 seconds despite the fact that the inner layers of the composite were unlikely to be reached by the UV irradiation.9 The optical pyrometry results indicated that the heat released during the photopolymerization helped to achieve full conversion of the epoxy groups. The modulus of these glass-fiber reinforced composites were as high as 24500 MPa when the glass fiber content was 70 % (Figure 3). According to DMA analysis the Tg of the composites were 110°C. The flexural resistance displayed by the GFREC was 1045 MPa when a trifunctional thiol was used in comparison with a value of 1232 MPa for the tetrafunctional thiol, as a result of the higher degree of crosslinking achieved. Therefore this method could be a more economic and efficient option to cure GFREC instead of the thermal curing method.
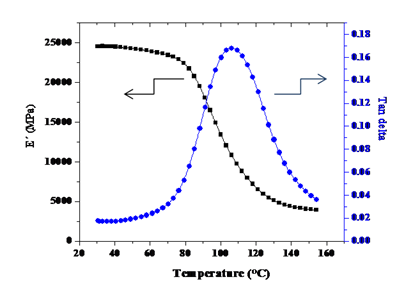
Figure 3 Storage modulus and tan delta of the prepared GFREC with 68% of glass fiber. Reproduced with permission of Springer, Figure 6 of reference 9.
Shape Memory Materials (SMP)
The presence of the flexible polythioethers in the co-network with the polyethers derived from the epoxy resin, resulted in a material with shape memory properties.10 The polythioethers derived form the thiol-ene system were in the “soft- phase” which induced this shape memory phenomenon. It was found that the polymer obtained by adding 10 mol % of the thiol-ene system to the epoxy resin was deformed when heated above the Tg (87°C) and the form preserved by cooling at 4°C, The polymer was recovered in its original form in13 seconds when heated above its Tg (Figure 4). It is worth mentioning that the storage modulus of these materials were as high as 3500 MPa. When the concentration of the thiol-ene system was augmented to 20 mol % the polymer recovered the original shape in 27 seconds because of the increased amount of the flexible polythioethers, reducing in this way the stress in the hard phase of the co-network, which is the driving force to recover the original form of the deformed polymer. The polymer was cured with 10 mol % of the thiol-ene system at 85°C and 40 mW/cm2 of UV light instensity, during 400s. The polymers were deformed: A) when heated at 90°C and the form preserved by cooling the polymer at 4°C. The original form was recovered B) when the sample was heated at 90°C.
Self-healing Epoxy Polymer
Self-healing properties are highy desirable in a material whether it is ceramic, metal or polymer. Both extrinsic and intrinsic method have been tested.11 One of the intrinsic approaches to achieving self healing properties is by introducing functional groups in the polymer that can undergo dynamic Exchange reactions between reversible covalent bonds, Several photochemical reactions have been used such as the Diels-Alder reaction, cycloaddition reaction [2+2], cycloadditions [4+4], exchange reaction of siloxanes segments, and disulfide exchange to name a few examples.12 In our epoxy/thiol-ene systems, given that a tetrafunctional thiol was used, it was thought that disulfide bonds could be introduced in the polymer if the tetrafunctional thiol was partially oxidized, generating an oligomer with both thiol and disulfide bonds.13 This way, the thiol groups could react with the double bonds of the curing agent inducing the anionic polymerization of the epoxy resin and at the same time introducing disulfide bonds in the resulting polymer. The best method to obtain the desired thiol-disulfide oligomer was by oxidizing the PTKMP with iodobenzenediacetate in a 1:1 stoichiometry relationship.
An oligomer with a molecular weight of 1837 daltons was obtained. The number of functional groups of this oligomer was determined by 1H NMR which found 8 thiol groups and 4 disulfide bonds in the chemical structure (Figure 5). When the formulation that included DGEBA, ALA4 and the oxidized oliogmer was photopolymerized, in the same conditions described above for the conventional epoxy/thiol-ene system, a rigid polymer with a Tg of 73°C was obtained. The test specimens with a 2 mm thickness were cut in half and then heated at different temperatures until the two pieces were joined into one piece. It was determined that the Polymer was completely healed in 10 minutes when heated at 80°C or for 500 minutes at room temperature (Figure 6). Further, it was determined through DMA that the healed specimen retained the mechanical properties in comparison with the control specimen. It is important to note that it was posible to get the self-healing epoxy polymers derived from the epoxy resins DGEBA, despite the rigidity of the specimen that displayed a Tg of 73°C.

Figure 5 Chemical structure of the obtained thiol-disulfide oligomer responsible for the self healing properties in the polymer derived from the epoxy/thiol-ene system.
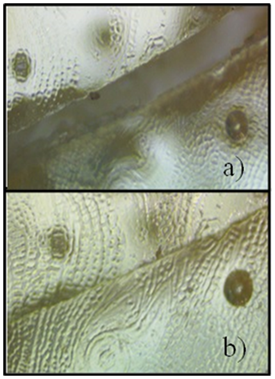
Figure 6 Photographs of the self-healing process of the test specimen derived from the anionic epoxy /thiol-ene system, A) after the specimen was cut in half, B) after the self-healing process at 70°C.
Epoxy Foams
Another direct application of the anionic epoxy/thiol-ene system is the preparation of epoxy foams, just by adding a chemical foaming agent. Though several foaming agents were tested, only the benzenesulfonyl hydrazide (BSH) was able to produce the epoxy foams. The foaming process was influenced by the concentration of the thiol-ene system (20-40 mol %) in the epoxy formulation. At a higher concentration of the thiol-ene system there was a dilution effect reducing the viscosity of the epoxy resin, producing epoxy foams with smaller pore size. The density of the epoxy foams was in the range of 366 Kg/m3, 788 Kg/m3. Figure 7 shows a photograph of the foamed test specimens with different concentrations of the thiol-ene system. The specimen with 20 mol% of the thiol-ene systems showed bigger pore size while the specimen with 40 mol% of the thiol-ene system displayed a more smaller and more uniform pore sizes.
This paper demonstrated the versatility of the photocurable anionic epoxy/thiol-ene. The high reactivity and the excellent mechanical properties of the derived polymers, allowed us to utilize this process in different applications such as GFREC, SMP, self-healing epoxy polymers and epoxy foams.
None.
The author declares no conflict of interest.

©2017 Acosta. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.