MOJ
eISSN: 2379-6383


Case Report Volume 11 Issue 1
1Halberg Hospital and Research Institute, Moradabad, India
2Asper Clinical Research Institute, St. Boniface Hospital, Winnipeg, Canada
3University Research Park, Kosice, Slovakia
4Comenius University, Bratislava, Slovakia
5School of Physiotherapy, Delhi Pharmaceutical Sciences and Research University Delhi
6Institute of Cardiovascular Sciences, Winnipeg, Canada
Correspondence: Viliam Mojto, Comenius University, Bratislava, Slovakia, Tel 00421905456834
Received: March 30, 2022 | Published: April 20, 2022
Citation: Singh RB, Tappia PS, Fedacko J, et al. Repeated episodes of physical training induced hypoxia, may be associated with improved exercise tolerance in covid-19. MOJ Public Health. 2022;11(1):50-52. DOI: 10.15406/mojph.2022.11.00374
Background: Repeated episodes of hypoxia by coronary artery ligation, precondition the myocardium to adapt against ischemic cardiac damage and arrhythmias. This case aims to highlight the role of hypoxia induced adaptation in exercise tolerance.
Case and methods: A male physician aged 77 years, presented with COVID-19 on April 17, 2021. Acute phase COVID-19 pneumonia, and lung fibrosis were diagnosed by high resolution computerized tomography and chronic hypoxia, measured by oximeter (SpO2 saturation between 91%-93%). Regular physical training in the form of slow jogging, morning, and evening, was advised twice daily.
Results: Treatment with physical training was associated with improved SpO2 saturation during exercise, from 83-84% to 89-91%. There was a significant increase in oxygen saturation during rest after treatment with physical training for two weeks. It is possible that repeated episodes of hypoxia during physical training, may have induced molecular adaptations in the heart and lungs, leading to increased exercise tolerance with increase in SpO2 saturation.
Conclusions: Regular physical training in the form of jogging may be associated with improvement in exercise tolerance without causing hypoxia. There is no other study in humans, to our knowledge, that has examined the role of physical training induced hypoxia to achieve myocardial adaptations, characterized with improved SpO2 saturation.
Keywords: inflammation, exercise, hypoxia, adaptation, physical training
Weakness, fatigue, insomnia and breathlessness are common clinical manifestations of acute COVID-19.1 Physical training as therapeutic intervention, has been shown to improve alertness in young, healthy sleep deprived individuals, and has been found to result in a more robust stimulation of neuronal metabolism, among healthy sleep deprived subjects.2–5 Apart from these benefits, physical training can also lower fatigue in patients with COVID-19 infection. However, many patients recovering from acute respiratory failure may terminate in chronic respiratory failure, due to the development of lung fibrosis, after pneumonia, due to COVID-19. The SpO2 saturation may vary between 90 to 94% which may worsen, resulting in hypoxia, on routine physical activity such as walking, taking bath or going to toilet. Repeated episodes of hypoxia by coronary artery ligation, can precondition the myocardium to adapt against ischemic cardiac damage and arrhythmias.6 Our hypothesis is that physical training may improve oxygen saturation, because repeated episodes of hypoxia during physical training may induce molecular adaptations in the heart and lungs,7–9 leading to increased exercise tolerance with increase in oxygen saturation. No study has examined the role of physical training showing increased oxygen saturation, among patients with hypoxia or border line reduced oxygen saturation, in COVID-19. The paucity of knowledge, prompts us to report this novel discovery in a patient, that physical training may induce repeated episodes of hypoxia, leading to improved exercise tolerance with greater SpO2 saturation.
A male physician aged 77 years, presented with COVID-19 on April 17, 2021. Further details of this patient regarding clinical presentation and treatment given in acute stage have been reported earlier.10 After recovery from acute phase COVID-19 pneumonia, this patient developed chronic hypoxia with SpO2 saturation between 91%-93%.
Past and family history revealed no significant antecedent. Physical examination showed that his pulse was 82/min, blood pressure 130/80 mm Hg, breathing at rest 17 per minute. Physical systemic examination revealed no abnormality in chest, abdomen, and cardiovascular system; his electrocardiogram and 2D echocardiography showed no abnormality (ejection fraction >65%).
In the first week of June 2021 (6 weeks later from acute phase), his total and differential blood counts, as well as platelet counts, were within normal limits. His blood lymphocytes were 37%/cmm, neutrophil 59% cmm, Hb 11.2g/dl. SGOT 42.0 IU/L (normal up to 0-40 IU), ferritin 305.60 ng/ml. His C-Reactive protein (CRP) was 7.41 mg/L (normal, 00.0 to 0.6 mg/L), D Dimer 248.2 ng/ml (normal <500ng/ml), IL-6, 4.21pg/ml (normal 0.0 to 0.5pg/ml). His LDH was also higher indicating increased inflammation and cardiac damage (250 IU, normal <225 IU). His computerized tomography of lungs with high resolution, showed haziness in lower lung zones, indicating post-COVID-19 fibrosis.
Treatment
He was administered nebulization with asthalin + budicort +evolin continuously and regularly twice daily. There was marked recovery from acute phase, after 6 weeks of treatment. Since he had persistent lungs’ fibrosis, he was administered tablet nintedanib soft gelatin capsules, 100 mg twice daily, and rosuvastatin 5 mg daily, without any improvement in oxygen saturation.
He was advised physical training in the form of slow jogging; 100 steps in approximately 2 minutes, intermittently for three times, after 3-5 min rest each time, in the morning and evening. However, he could not do any exercise due to marked decline in Sp2 levels down to 81-84%. Therapeutic intervention with physical training was associated with significant changes in the SpO2 measured via oximeter as well as multipara monitor. At the baseline, physical exercise was associated with modest decline in SpO2 down to 81% to 84% during jogging, which would recover on taking rest, during the next 3-5 minute. However, after exercise training for the next two weeks, there was a significant improvement in exercise tolerance Table 1, Figure 1, 2. The effects of exercise training on SpO2 with reference to hypoxia, which is clearer in Figure 2. There is a marked increase in exercise tolerance after physical training at 0, 1, and 5 minutes.
Day of therapy |
During exercise, before training, (SpO2 %) |
After 1 min (SpO2 %) |
After 5 min (SpO2 %) |
Day 1 |
83 |
87 |
90 |
Day 2 |
85 |
88 |
90 |
Day 3 |
84 |
89 |
90 |
Day 4 |
85 |
88 |
90 |
Day 5 |
86 |
90 |
91 |
Mean* ± SD |
84.6 ± 1.14 |
88.4 ±1.14* |
90.2 ±0.44* |
AFTER |
PHYSICAL |
TRAINING |
|
Day 1 |
88 |
92 |
94 |
Day 2 |
89 |
93 |
94 |
Day 3 |
90 |
93 |
95 |
Day 4 |
91 |
93 |
94 |
Day 5 |
92 |
93 |
95 |
Mean* ± SD |
90.0 ±1.58 |
92.8 ±0.44* |
94.4 ±0.55* |
Table 1 Effects of exercise training on oxygen saturation with reference to hypoxia
*=Mean ± standard deviation of highest values. *= p<0.01, values were obtained by comparison of data after 1, and 5 minutes with data obtained during exercise. Statistical differences were analyzed using repeated measure one-way Analysis of Variance (ANOVA), followed by Tukey’s Multiple Comparison Test using GraphPad Prism Software, ver. 8.0.2.
This case report reveals that physical training such as slow jogging may be associated with significant increase in SpO2, in a patient with post-acute COVID-19 having subacute to chronic hypoxia Table 1, Figure 1. The findings reveal that treatment with physical training also allowed increased exercise tolerance from hypoxia to near normal oxygen saturation Table 1, Figure 2. Since, no such evidence exists in human beings, therefore, direct comparisons between this case report and the established literature are not possible.
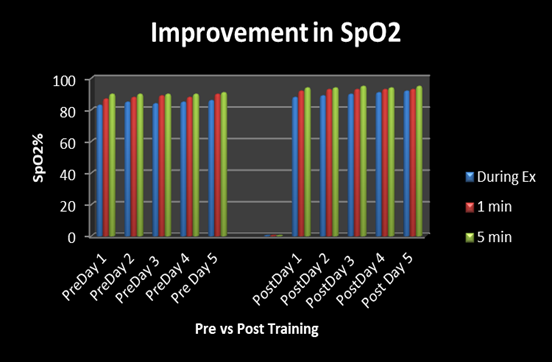
Figure 1 Bar diagram showing significant increase in oxygen saturation after 1 min, and 5 min. Comparison of SpO2 saturation during exercise before and after physical training.
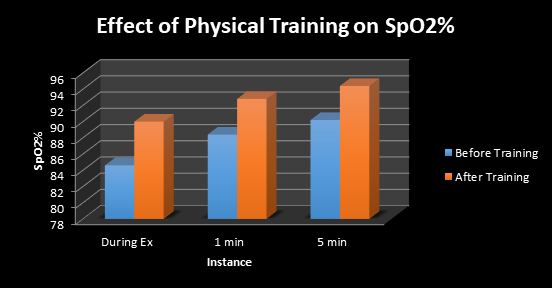
Figure 2 Effect of physical training on SpO2 at baseline, before training and after training of 4 weeks, showing significant increase in exercise tolerance. Ex=Exercise.
There is evidence that immobilization due to hospitalization and bed rest with decreased physical activity due to sustained quarantine and social distancing, can downregulate the ability of body organ systems. Hence patient’ immune system is not able to resist to complications of viral infection and increase the risk of damage to the immune cells as well as respiratory, cardiovascular, musculoskeletal systems and the brain.
Repeated episodes of hypoxia can increase the intrinsic capability of the myocardium and possibly alveolar cells to adapt, providing protection against arrhythmias and ischemia. This poses the possibility that our human experiment may be the first to observe that repeated stimulation via physical training induced hypoxia may cause increased exercise tolerance. However, repeated episodes of hypoxia may be an assumption, because performing repeated episodes of hypoxia, may have an impact of repeated exercise training on SpO2 levels.11-13
There is evidence that acute hypoxia via stimulation of the β adrenergic receptors can occur, causing increase in the heart rate and the cardiac output. Having increased the systolic blood pressure and affected the heart rate, it tends to accelerate the Rate Pressure Product. However, if it persists for a prolonged period, it may induce ischemia and cardiac hypertrophy which may in due course of time cause heart failure. Evidence states that chronic hypoxia leads to several adaptive changes, that sustains function with heart tissue oxygen consumption at ~0.1 ml O2/g/min, which is approximately 6ATP consumption per day. The metabolic adaptations induced by chronic hypoxia may thus lead to efficient utilization of limited oxygen supply and preserve function by alternatively shifting to glucose metabolism. Though the mechanism for this remains unexplored, experiments suggest that training adaptations induced by exercise may alter cardiac gene expression and signaling and may also reduce or abate oxidative stress and inflammation as well as apoptotic activity.
Since hypoxemia may be associated with potential negative effects, the physician should be aware of the accuracy of these instruments. The accuracy of pulse oximeter findings obtained during exercise has been described, but without any consistent findings, which indicate a weakness of our study.
It seems that regular physical training in patients with hypoxia, may improve SpO2 and produce increased exercise tolerance against hypoxia, possibly due to molecular adaptations in the myocardium and the lungs. Large randomized, controlled trials are necessary to confirm our findings.
None.
The authors declare no conflict of interests.
Written informed consent was obtained from the patient. A copy of the consent is available for review by the Editor of this journal.

©2022 Singh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.