MOJ
eISSN: 2379-6383


Research Article Volume 11 Issue 1
1Le Majoral Building 34420 Portiragnes Plage, France
2Organization of Cooperation and Coordination for Endemic Diseases Control in Central Africa (0CEAC) PO 288 Yaoundé Cameroon
3Malaria Control Programme, Sonamet, Lobito, Angola
4HSM, Univ. Montpellier, CNRS, IRD, Montpellier, France
Correspondence: Carnevale P, Le Majoral Building 34420 Portiragnes Plage, France
Received: September 21, 2021 | Published: March 23, 2022
Citation: Carnevale P, Carnevale N, Toto JC, et al. Long-term evolution of Plasmodium falciparum gametocytes index during a village scale malaria vector control program comparing durable lining, long-lasting treated net and indoor residual spraying in Angola. MOJ Public Health. 2022;11(1):17-26. DOI: 10.15406/mojph.2022.11.00369
Rationale: Due to the serious side effect of some anti-gametocyte drugs on G6PG efficiency carriers, a special attention was given to the possible influence of vector control on the evolution of P. falciparum gametocyte index (GI) in the framework of the long-term village scale malaria vector control project implemented in 8 villages around the Balombo town (Angola). The aim of the project was to compare, in paired villages, the epidemiological efficacy of Long lasting deltamethrin Insecticide Treated Nets (δLLIN), lambdacyhalothrin Inside Residual Spraying (ʎIRS), and deltamethrin Insecticide Treated Plastic Sheeting (δITPS) applied on the indoor walls of houses, used alone, or associated with δLLIN or after the 2 rounds of λIRS.
Methods: The program started in 2007 and full vector control (VC) implementation was done in December 2008 owing two years of baseline data collection in the 8 selected villages around Balombo city. Parasitological evaluation was based upon regular cross-sectional surveys (CSS) on randomized samples of population for each survey, focusing ≤ 15 years children, to compare GI before/after and here/there according to each VC method. Field made thick blood smears (TBS) were microscopically examined by the same team of the Medical Department of the Angolese Sonamet Company, which supported the trial, with 10% of randomized slides double-checked in OCEAC, Yaoundé (Cameroon).
Results: 234 cross-sectional surveys were carried between 2007 and 2018. P. falciparum gametocytes were observed in 574 of the 23,822 thick blood smears examined for a GI of 2.41%. During the 3 years following the VC full implementation, the GI decreased by 71%, from 4.05% (n= 6,697) before VC to 1.17% (n= 7,667 after VC) with similar influence of each VC method. During the 5 following years GI remained at a very low level (0.23%; n=2,992). Due to the national malaria outbreak, an increase was noticed after 2015. The general CSS survey done in February 2018, 11 years after the first one (February 2007), showed in the same 5 villages a significant 80% decrease of GI, from 6.98% (n=773) to 1.42% (n=421).
Conclusion: The trial showed that a long-term malaria village scale vector control program had a long lasting and significant impact on P. falciparum gametocytes and the influence of VC on GI could therefore be recommended as another relevant objective of the National Malaria Control Program.
Keywords: P. falciparum, gametocyte index, long-term evaluation, vector control, LLIN, ITPS, IRS, angola
As early as 1900, it was known that the human being with crescents forms of the parasite in the peripheral blood was infectious for the Anopheles vector,1-5 and a new avenue for malaria control opened up by targeting gametocytes, their absence meaning no transmission,6–8 while maintaining Anopheles mosquitoes in the natural trophic chain supporting the ecological and epidemiological interesting situation of anophelism without malaria.
However, the problems arise with some anti-gametocyte drugs, such as primaquine (PQ), which can have a serious impact (hemolysis) on G6PD deficiency6 carriers and it was not certain that adding PQ to treatment regimens for patients with Plasmodium falciparum infection would reduce malaria transmission. In individual patients, it reduces gametocyte prevalence and density. In practical terms, even if PQ results in large reductions of gametocytes in people being treated for malaria, there is no reliable evidence that this will reduce transmission in a malaria-endemic community where many people are infected but have no symptoms and are unlikely to be treated.6 Indeed, the absence of any symptoms in gametocyte carriers makes their detection difficult and thus the actual evaluation of the infectivity of the human population for local vectors.
The probability that an Anopheles vector will make a full Plasmodium sporogonic development after feeding on a gametocyte carrier depends on several parameters including the prevalence, duration of presence, density, viability, maturity, sex-ratio of gametocytes as well as other factors affecting their transmissibility.9–16
On the other hand, the importance of gametocytocide activity of some drugs in the selection and propagation of resistant strains is discussed. For Barnes and White,9 gametocyte carriage and infectivity to mosquitoes is consistently higher in patients infected with drug resistant compared with drug sensitive malaria parasites, which would participate in the expansion of resistant strains. This would be, for example, for resistance to Sulfadoxine-Pyrimethamine (SP) resulting from a prevalence and high density of gametocytes following conventional treatment; the use of chloroquine and SP would increase the carriage of gametocytes.17–19
In South Africa, the SP treatment initiated in Mpumalanga Province has been evaluated twice annually.20 It then appeared that the treatment was efficient, but an increased duration and density of gametocyte carriage after SP treatment was an early indicator of drug resistance. This increased gametocytaemia among patients who have primary infections with drug-resistant P. falciparum fuels the spread of resistance even before treatment failure rates increase significantly.
Hence the interest of drugs combining schizonticide and gametocytocide effects, such as artemisinin derivative associated with a schizonticide with a long half-life, is now commonly referred to as Artemisin-based combination therapies (ACT). The artemether-lumefanthrin combination has been described as reducing the carriage of gametocytes.21 In areas of low transmission, large-scale use of ACTs has resulted in reduced transmission and resistance, and routine use of ACTs should be recommended to treat disease and prevent transmission. These clinical and parasitological problems of an anti-gametocyte drug raise the interest of another possibility to reduce the parasite reservoir using vector control.
The initial objective of the long term, village scale, malaria vector control project carried out around Balombo (Benguela Province, Angola), was to assess and compare the evolution of the prevalence, incidence and density of Plasmodium infections of symptomless carriers (or asymptomatic), according to each vector control method implemented.22 The goal of this work was not to study either the seasonal dynamics, density, sex-ratio of Plasmodium infections, or the infectivity of vectors. Therefore, the objective of this work was to analyze the evolution of the percentage of P. falciparum gametocyte carriers according to the implementation of each vector control method against the main local vectors, An. funestus and An. gambiae.
The description of the study area and the protocol of vector control operations were already presented.23, 24 Four vector control methods were implemented including (1) Perma®Net 2.0 ("P2") long-lasting deltamethrin insecticide impregnated mosquito nets (“δLLIN”) alone, distributed in 2 villages (Caala and Cahata) in 2 steps: first, at least one mosquito net / house provided in February 2007, then one mosquito net / sleeping unit supplemented in February 2008, with a completed coverage in December 2008. (2) “P2” impregnated mosquito net associated with deltamethrin insecticide treated plastic sheeting (“δITPS”) (“Zero Fly©” model) pinned on the walls in every house in 2 villages, Capango and Canjala, provided in December 2008. (3) Deltamethrin treated ITPS (“δWall Lining” model) alone, pinned on the walls in sleeping areas of every house in 2 villages, Barragem and Chisséquélé, installed in December 2008. (4) Indoor residual spraying (λIRS) with lambdacyhalothrin, 2 rounds done in December 2008 and the second in June 2009, in 2 villages, Candiero and Libata, then installation of δITPS in January 2010.
Parasitological surveys were based on the classical, regularly done, cross-sectional surveys (CSS) on randomized samples, at each survey, of the populations, from the previous numbering of every house for further mapping analysis. Thick blood smears (TBS) prepared during each CSS, were immediately stained (Giemsa), then microscopically examined (Light Optical determination) in the medical service of the Angolan company Sonamet© in Lobito with a double-checking of 10% of the samples at the Parasitology Department of the Pan-African Organization OCEAC in Yaoundé, Cameroon.
To compare the situation and their evolution with the vector control operations, the age group ≤ 15 years was retained in the different statistical analysis.24
Actually a preliminary analysis of the data collected during the first two years (2007-2008) in two control villages, Capango and Canjala (no vector control implemented), indicated statistically comparable gametocyte index in the main age groups conventionally considered in malariology such as 0-2 years, 3-5 years, 6-9 years and 10-15 years; respectively 5.32% (n=413), 5.09% (n=628), 5.48% (n=657), and 3.35% (n=418). It was therefore possible to consider one single age group only (≤15 years) to improve the power of statistical analysis of the gametocyte index recorded during the study.
For each sample, name, age and gender of the patient were noticed and 4 indicators were considered: Plasmodium species, prevalence and density of trophozoites and P. falciparum gametocytes (gametocyte index).
The whole Balombo project included 3 successive phases: a "short-term" evaluation in the 8 villages, between 2007 and 2011, therefore 2 years before and 3 years after the implementation of each vector control; a "medium-term" evaluation in 4 villages: one per control method with a 10 years monitoring; and a “long-term” evaluation with a final CSS carried out in February 2018, involving (for operational issues) 5 of the 8 initial villages to evaluate the parasitological situation 11 years after the beginning of the first operations. Several other interventions were locally made by different actors, such as the distribution of impregnated mosquito nets during antenatal consultations in Balombo Hospital as part of the National Malaria Control Program, distribution of different models of impregnated mosquito nets by various NGOs, larval control with Bacillus thuringiensis by Cuban teams, etc. It was not possible to obtain reliable information on these actions, but they were implemented in the different villages and therefore the comparison of situations in the villages of the project, and their evolution, remain relevant.
The comparison of percentages was based on Chi square and Odds Ratio (OR) (with their Lower and Upper Limits) with Epi info version 7.2 software.25
A total of 234 cross-sectional surveys (CSS) were carried out from February 2007 to February 2018 and P. falciparum gametocytes were observed in 574 of the 23,822 thick blood smears of children ≤15 years old, i.e., a general gametocyte index of 2.41% with large variations in space (villages) and time.
III-1. Short-term evaluation (5 years): 2007-2011
During the first 5 years of the trial, P. falciparum gametocytes were microscopically detected in 549 of the 20,186 thick smears examined, i.e., a general gametocyte index of 2.72% with a relatively small variation according to villages Figure 1. The striking drop of 60%, which occurred in 2009 Figure 2 corresponds to the implementation of vector control operations in December 2008, and must be underlined.
III-1-1. Natural evolution of gametocyte index in the 6 control villages in 2007-2008, before vector control.
The results of surveys carried out in 2007 and 2008 in the 6 villages before vector control implementation are gathered in Table 1. Three situations were noticed, some increase, but not significant (Barragem, Candiero), similar level (Canjala, Libata), and a significant decrease in Capango (from 6.91% to 2.36%; χ2 = 8.08; p <0.05; OR = 0.33 [0.13-0.77]) and in Chisséquélé (from 7.00% to 2.94%; χ2 = 9.04; p = 0.0026; OR = 0.40 [0.21-0.77]) Table 1, Figure 3. For the 6 control villages, considered altogether, the average gametocyte indexes recorded in 2007 and 2008 were comparable: respectively 4.38% (n = 2,650) and 3.83% (n = 4,047) (χ2 = 1.24; P = 0.27; OR = 1.15 [0.89-1.48]).
Villages |
Year 2007 |
Year 2008 |
Difference |
χ2 (P-value) |
OR |
Capango |
6.91% |
2.36% |
-66% |
8.08* |
0.33 |
(n=275) |
(n=381) |
(P=0.0042) |
[0.13-0.77] |
||
Canjala |
5.30% |
5.11% |
-3% |
0.02 |
0.96 |
(n=661) |
(n=801) |
(P=0.88) |
[0.59-1.57] |
||
Chisséquélé |
7.00% |
2.94% |
-58% |
9.04* |
0.4 |
(n=357) |
(n=646) |
(P=0.0026) |
[0.21-0.77] |
||
Barragem |
1.40% |
2.57% |
83% |
1.69 |
0.54 |
(n=428) |
(n=622) |
(P=0.19) |
[0.19-1.48] |
||
Candiero |
2.96% |
4.53% |
53% |
1.79 |
0.87 |
(n=439) |
(n=751) |
(P=0.18) |
[0.47-1.59] |
||
Libata |
3.67% |
4.26% |
16% |
0.24 |
0.86 |
(n=490) |
(n=846) |
(P=0.63) |
[0.46-1.58] |
||
Total |
4.38% |
3.83% |
-12,5% |
1.24 |
1.15 |
(n=2650) |
(n=4047) |
(P=0.27) |
[0.89-1.48] |
Table 1 Natural evolution of P. falciparum gametocyte index in control villages before implementation of vector control.*, Statistically significant differences, OR= Odds ratio, with Lower and Upper limits
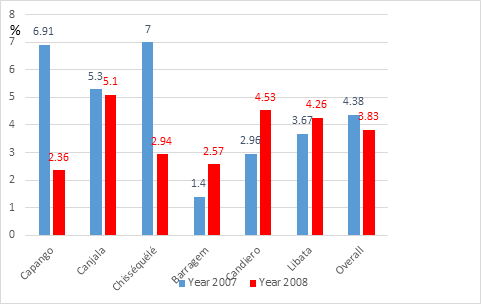
Figure 3 Natural evolution of P. falciparum gametocyte indexes in the 6 control villages before vector control implementation.
III-1-2. Evolution of gametocyte indexes in the 6 control villages between 2009 and 2011, after the implementation of vector control
The results of the surveys carried out in the 6 villages in 2007-2008 (= before) and 2009-2010 and 2011, (= after the implementation of the various vector control operations), are gathered in Table 2.
Villages |
Before VC |
After VC |
Difference |
χ2 |
OR |
Capango* |
4.27% |
1.22% |
-71.40% |
16.19 |
0.28 |
(n=656) |
(n=1,064) |
[0.14-0.56] |
|||
Canjala* |
5.20% |
1.85% |
-64.40% |
22.09 |
0.34 |
(n=1,462) |
(n=1,298) |
[0.21-0.56] |
|||
Chisséquélé* |
4.33% |
0.94% |
-78.50% |
27.9 |
0.21 |
(n=1,003) |
(n=1,278) |
[0.10-0.41] |
|||
Barragem |
2.10% |
1.27% |
-39.50% |
2.37 |
0.6 |
(n=1,050) |
(n=1,255) |
(p=0.12) |
[0.30-1.20] |
||
Candiero* |
3.95% |
0.78% |
-80.30% |
29.91 |
0.19 |
(n=1,190) |
(n= 1,416) |
[0.09-0.38] |
|||
Libata* |
4.04% |
1.03% |
-74.50% |
24.75 |
0.25 |
(n=1,336) |
(n=1,356) |
[0.13-0.46] |
|||
Total* |
4.05% |
1.17% |
-71.10% |
128.4 |
0.28 |
(n=6,697) |
(n=7,667) |
[0.22-0.36] |
|||
Table 2 P. falciparum Gametocyte indexes in control villages before and after vector control implementation.*, Statistically significant differences, VC= vector control, OR= Odds ratio, with Lower and Upper limits
Excepted in Barragem, the decrease of gametocyte indexes was significant in every villages Figure 4 and for the 6 control villages the overall index significantly dropped from 4.05% (n = 6,697) to 1.17% (n = 7,667) (χ2 = 128.4;
OR= 0.28 [0.22-0.36]), i.e. a 71% reduction of gametocyte carriers Table 2 during this period.

Figure 4 Evolution of P. falciparum gametocyte index in each village before/after vector control implementation.
The evolution of the gametocyte indexes induced by each control method are gathered in Table 3 which shows that the three methods were remarkably efficient in significantly reducing the percentages of subjects with gametocytes detected by conventional light microscopy (LM) Figure 5.
Method of vector control |
Gametocyte |
Indexes |
Difference |
χ2 |
OR |
Before |
After |
||||
LLIN+ZF |
4,91% |
1,57% |
-68% |
40,9 |
0,31 |
(n=2,118) |
(n=2,362) |
[0,21-0,46] |
|||
ITPS |
3,21% |
1,10% |
-66% |
25,1 |
0,34 |
(n=2,053) |
(n=2,533) |
[0,21-0,54] |
|||
IRS |
3,39% |
0,90% |
-73,5% |
54,9 |
0,22 |
(n=2,526) |
(n=2,772) |
[0,14-0,35] |
|||
Total |
4,05% |
1,17% |
-71% |
120,4 |
0,28 |
(n=6,697) |
(n=7,667) |
[0,22-0,36] |
Table 3 Evolution of P. falciparum gametocyte indexes according to the method of vector control implemented (LLIN+ZF= long-lasting treated nets + Zero Fly; ITPS= insecticide treated plastic sheeting alone; IRS= indoor residual spraying)

Figure 5 Evolution of P. falciparum gametocyte indexes after implementation of each vector control method.
III-1-3. Gametocyte index in the 2 villages with impregnated mosquito nets: Caala and Cahata.
The results of the 10 surveys carried out in 2007 and the 12 surveys carried out in 2008 in these 2 villages are gathered in the Table 4a.
Year 2007 |
Year 2008 |
Total |
|||||||
Param. |
G+ |
n |
% |
G+ |
n |
% |
G+ |
n |
% |
Caala |
27 |
802 |
3.37 |
35 |
888 |
3.94 |
62 |
1,690 |
3.67 |
Cahata |
35 |
738 |
4.74 |
36 |
666 |
5.4 |
71 |
1,404 |
5.06 |
Total |
62 |
1,540 |
4.03 |
71 |
1,554 |
4.57 |
133 |
3,094 |
4.3 |
Table 4a P. falciparum gametocyte carriers in the 2 villages having received LLIN since February 2007 (G+, number of thick smears with gametocytes; n, number of thick smears examined; param., parameters)
Year 2007 |
Year 2008 |
χ2 (P value=) |
OR |
|
Caala |
3,37% |
3,94% |
χ2=0,39 |
0,85 |
(n=802) |
(n=888) |
(P=0,54) |
[0,49-1,46] |
|
Cahata |
4,74% |
5,40% |
χ2=0,32 |
0,87 |
(n=738) |
(n=666) |
(P=0,57) |
[0,53-1,44] |
|
χ2 |
1,88 |
1,87 |
||
(P=) |
(P=0,17) |
(P=0,17) |
||
OR |
0,70 |
0,72 |
||
[0,41- 1,29] |
[0,43-1,19] |
|||
Table 4b Statistical analyses of P. falciparum gametocyte indexes in the 2 villages which received LLIN in February 2007 (OR, Odds Ratio)
The statistical analyses are reported in the Table 4b.
In 2007, after the first distribution of impregnated mosquito nets, the gametocyte indexes were comparable in the two villages: respectively 3.37% (n = 802) and 4.74% (n = 738) in Caala and Cahata (χ2 = 0.39; P = 0.54; OR = 0.85 [0.49-1.46]). In 2008, after the second distribution of LLIN, the gametocyte indexes were also comparable in the two villages, respectively 3.94% (n = 888) and 5.40% (n = 666) in Caala and Cahata (χ2 = 0.32; P = 0.57; OR = 0.87 [0.53-1.44]). For these two years the gametocyte indexes did not significantly change neither in Caala (from 3.37% to 3.94%) nor in Cahata (from 4.74% to 5.40 %) Figure 6a in spite of the increased number of impregnated mosquito nets provided. The gametocyte indexes reported for 2007 and 2008 in these two villages with LLIN (4.30%; n = 3094) Table 4a and in the 6 control villages (4.05%; n = 6697) Table 3 were similar (χ2 = 0.34; P = 0.56; OR = 1.07 [0.86-1.32]).
For the 3 years corresponding to the period after vector control in the 2 villages with LLIN, the gametocyte indexes significantly dropped from 4.30% (n= 3,094) before VC to 2.02% (n = 2,728) after VC, (χ2 = 24.2; OR = 0.46 [0.33-0.64]) Table 4c, i.e. a 53% decrease. The sharp drop in 2009 (from 4.6% in 2008 to 2.0% in 2009) following the full coverage in LLIN is noteworthy Figure 6a. In the 6 control villages, the gametocyte index significantly dropped from 4.05% to 1.17% Table 3 and was significantly lower than the gametocyte index of the 2 LLIN villages (χ2 = 10.4; P = 0.0013; OR = 0.58 [0.41-0.82]). Therefore, in 3 years after vector control, gametocyte indexes were reduced by # 50% in the 2 villages with LLIN and by # 70% in the 6 villages with the 3 other methods Figure 6b.
Years |
Parameters |
Caala |
Cahata |
Total |
G+ |
14 |
11 |
25 |
|
2009 |
n |
716 |
534 |
1,250 |
% |
1.96 |
2.06 |
2 |
|
G+ |
17 |
6 |
23 |
|
2010 |
n |
639 |
580 |
1,219 |
% |
2.66 |
1.03 |
1.89 |
|
G+ |
1 |
6 |
7 |
|
2011 |
n |
134 |
125 |
259 |
% |
0.75 |
4.8 |
2.7 |
|
G+ |
32 |
23 |
55 |
|
Total |
n |
1,489 |
1,239 |
2,728 |
% |
2.15 |
1.86 |
2.02 |
Table 4c Evolution of P. falciparum gametocyte indexes in the villages with LLIN the 3 years, 2009-2011, after full coverage
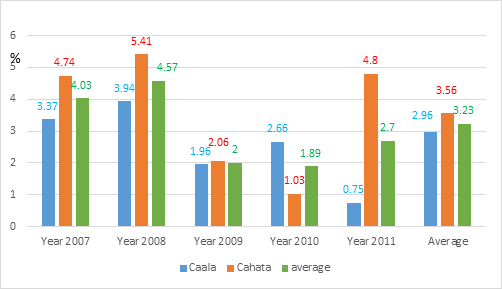
Figure 6a Evolution of P. falciparum gametocyte indexes in the 2 villages furnished in LLIN since 2007 (partial coverage) and with full coverage in 2008.
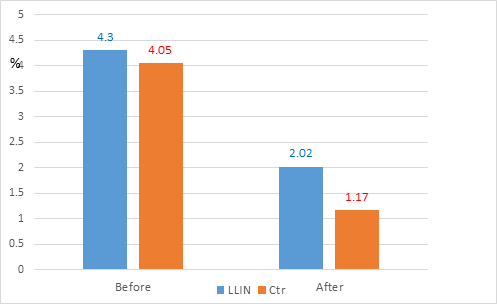
Figure 6b Overall evolution of P. falciparum gametocytes carriers with vector control implementation (before versus after) in LLIN and control villages.
III-2. Middle term evolution (2011-2016): longitudinal surveys in 4 villages: Cahata, Capango, Barragem, and Candiero.
During the 36 CSS longitudinal surveys carried out from 2011 to 2016 in 4 villages, (one by vector control method implemented, 9 surveys/village), 2992 thick blood smears were done and P. falciparum gametocytes were observed in 7 blood samples i.e., an overall gametocyte index of 0.23%, almost similar in the 4 villages (Table 5a) and 20 times less than the 4% observed at the beginning of the trial.
Villages |
No surveys |
No TBS*examined |
No TBS with gametocytes |
% |
Cahata |
9 |
933 |
4 |
0.43 |
(LLIN alone) |
||||
Capango |
9 |
619 |
1 |
0.16 |
(LLIN + ZF) |
||||
Barragem |
9 |
674 |
1 |
0.15 |
(ITPS alone) |
||||
Candiero |
9 |
766 |
1 |
0.13 |
IRS then ITPS |
||||
Total |
36 |
2,992 |
7 |
0.23 |
Table 5a P. falciparum gametocyte index noticed between 2012 and 2016 in 4 villages each one having received one of the 4 different methods of vector control (* TBS, thick blood smears)
In the initially procured LLIN village, Cahata, the GI was similar to the GI in the initially 3 control villages (Capango, Barragem, and Candiero), respectively 0.43% (n= 933) and 0.15% (n=2,059) (corrected χ2= 0.158; P=0.282; OR= 2.96 [0.66-12.21]). The evolution of the gametocyte index each year during this period showed very low levels for 4 years (Table 5b) and 2 successive years with no gametocyte microscopically detected among the 1082 thick blood smears examined. It is worth underlying that this phase of particularly low gametocyte index was observed with each one of the 4 control methods implemented Figure 7.
Years |
No G+ |
No TBF |
% |
2012 |
1 |
1,223 |
0.082 |
2013 |
0 |
808 |
0 |
2014 |
0 |
274 |
0 |
2015 |
1 |
524 |
0.19 |
2016 |
5 |
163 |
3.06 |
Total |
7 |
2,992 |
0.23 |
Table 5b Overall P. falciparum gametocyte indexes noticed each year in the four villages longitudinally monitored. (No G+ = number of thick blood smears (TBS) with gametocytes)

Figure 7 Long term evolution of P. falciparum gametocyte indexes in the 4 villages with each vector control method.
III-3. Long term evolution: Overall cross-sectional final survey in the 8 villages, February 2018
In February 2018, a cross-sectional survey was carried out in the 8 initial villages and gametocytes were diagnosed in 18 of the 644 samples examined, i.e., an average gametocyte index of 2.79% Table 6 almost 2.5 times less than the 6.98% of the beginning of the trial in the same villages in February 2007. Comparing the gametocyte index of ≤ 15 children noticed for the same 5 villages in February 2007 and February 2018 (Table 6) shows that the average initial gametocyte index was significantly reduced from 6.98% (n=773) to 1.42% (n = 421) in 2018 (χ2 = 17.66; OR = 0.19 [0.07-0.47]), i.e. an average decrease of about 80% and such drop was observed in every village (-85% in Caala, - 71% in Cahata, - 81% in Capango, - 86% in Canjala, and - 73% in Libata) Figure 8. It is worth underlining the dynamic of the gametocyte index during this decade Table 7 with 3 periods: the sharp drop in 2009, the long-term low levels and the increase since 2015 Figure 9, which could be related to the malaria outbreak that occurred at the national and provincial scales.
Villages |
Feb-07 |
Feb-18 |
||||
n |
G+ |
% |
n |
G+ |
% |
|
Caala |
84 |
7 |
8.33 |
82 |
1 |
1.22 |
Cahata |
175 |
15 |
8.57 |
79 |
2 |
2.53 |
Capango |
79 |
5 |
6.33 |
83 |
1 |
1.2 |
Canjala |
215 |
17 |
7.91 |
93 |
1 |
1.08 |
Chisséquélé |
-- |
-- |
-- |
81 |
6 |
7.41 |
Barragem |
-- |
-- |
-- |
61 |
1 |
1.64 |
Candiero |
-- |
-- |
-- |
81 |
5 |
6.17 |
Libata |
220 |
10 |
4.55 |
84 |
1 |
1.19 |
Total |
773 |
54 |
6.98 |
644 |
18 |
2.79 |
Table 6 Comparison of P. falciparum gametocyte index noticed in February 2007 (= before vector control) and February 2018 (11 years after full vector control implementation) in children ≤15 years old
nb TF |
G+ |
% |
|
Year 2007 |
1880 |
73 |
3,88% |
Year 2008 |
2420 |
95 |
3,93% |
Year 2009 |
2187 |
36 |
1,65% |
Year 2010 |
2267 |
21 |
0,93% |
Year 2011 |
520 |
6 |
1,15% |
Year 2012 |
1223 |
1 |
0,08% |
Year 2013 |
808 |
0 |
0,00% |
Year 2014 |
274 |
0 |
0,00% |
Year 2015 |
524 |
1 |
0,19% |
Year 2016 |
163 |
5 |
3,07% |
Year 2018 |
304 |
9 |
2,96% |
Sum |
12570 |
247 |
1,96% |
Table 7 Yearly evolution of P. falciparum gametocyte index in the 5 villages monitored from 2007 to 2018 (No TBS= number of thick blood smears examined; G+ = number of TBS with P. falciparum gametocytes)
|
Villages |
Before VC |
After VC |
Stat. analysis |
|
Caala |
3.67% (n=1,690) |
2.14% (n=1,498) |
χ2=6.52 |
|
OR=0.57 [0.36-0.90] |
|||
|
Cahata |
5.02% (n=1,414) |
1.86% (n=1,239) |
χ2=19.35 |
|
OR=0.36 [0.22-0.59] |
|||
|
Capango |
4.46% (n=650) |
1.32% (n=1,060) |
χ2=16.21 |
|
OR=0.29 [0.14 0.57] |
|||
|
Canjala |
5.19% (n=1,463) |
1.93% (n=1,246) |
χ2=20.22 |
|
OR=0.36 [0.23-0.57] |
|||
|
Chisséquélé |
4.40% (n=999) |
0.94% (=1,278) |
χ2=28.07 |
|
OR=0.21 [0.10-0.41] |
|||
|
Barragem |
2.30% (n=1,042) |
1.27% (n=1,255) |
χ2=3.52 |
|
OR=0.55 [0.28-1.08] |
|||
|
Candiero |
3.68% (n=1,273) |
0.78% (n=1,416) |
χ2=26.83 |
|
OR=0.21 [0.10-0.41] |
|||
|
Libata |
4.05% (n=1,334) |
1.03% (n=1,355) |
χ2=24.79 |
|
OR= 0.25 [0.14-0.45] |
|||
|
Total |
4.12% (n=9,870) |
1.41% (n=10,347) |
χ2=139.70 |
|
OR=0.33 [0.27-0.40] |
|
Method of VC |
Before VC |
After VC |
Stat. Analysis |
|
LLIN |
4.28% (n=3,104) |
2.01% (n=3,737) |
χ2=24.17 |
|
OR=0.46 [0.33-0.64] |
|||
|
LLIN + ZF |
4.97% (n=2,113) |
1.65% (n=2,306) |
χ2= 38.85 |
|
OR=0.32 [0.22-0.47] |
|||
|
ITPS |
3.33% (n=2,041) |
1.11% (n=2,533) |
χ2= 27.26 |
|
OR=0.32 [0.20-0.52] |
|||
|
IRS then ITPS |
3.87% (n=2,612) |
0.90% (n=2,771) |
χ2= 51.7 |
|
OR=0.23 [0.14-0.36] |
Annex
Statistical analysis
Evolution of P. falciparum gametocyte index (VC, vector control; χ2, chi square; OR, Odds Ratio).
Gametocytes can be considered as a “weak link” in the epidemiology of malaria since without gametocytes there is no infected vector, therefore no new inoculations and new Plasmodium infections (incidence) leading to the elimination (as targeted) of malaria.26–28 Therefore, a great number of studies, old and recent, have been devoted to this stage of the biology of the Plasmodium,14,29–31 which is also the target of the “altruistic vaccine” (Transmission-blocking vaccines or TBV) aimed at the protection of the community.32 One approach to control gametocyte is the use of a gametocytocidal drugs such as primaquine (PQ),13,33–37 which has been associated with schizonticide for malaria crisis management but its impact on transmission is discussed, while mass treatment could increase the risk of selection of resistant strains. The side-effects of PQ on G6PD deficiency carriers are well known 6 and do not allow the larga manu use of this product, even in health centers not well equipped to detect this deficit.38
An analysis of 11 randomized trials including 1,776 P. falciparum infected patients treated with one of the following drugs, chloroquine (Cq), sulfadoxine-pyrimethamine (SP), quinine (QN), mefloquine (MQ), artesunate (AS), or different combinations (ACT) and a single dose of primaquine (PQ) was recently done (6). In patients who received a dose of primaquine, the prevalence and density of gametocytes was lower, but it was not clear whether PQ, added to treatment regimens for patients with P. falciparum infection, reduced transmission of malaria, particularly in endemic areas where many people are infected but have no symptoms and are unlikely to be treated. The recommended use of artemisinin-based combination therapy (ACT) for the treatment of malaria attacks may also have a negative effect on the sexual stages of Plasmodium,39,40 while the inverse impact has been observed.41 In Mali, a decrease in the infectivity of P. falciparum gametocytes towards Anopheles gambiae has been reported after treatment with sulfadoxine-pyrimethamine.42 The use of some drugs, such as piperaquine,43 chloroquine,31, 44,45 sulfadoxine-pyrimethamine46–48 may be of interest to control the asexual stages but they could stimulate the gametocytogenesis.
In The Gambia, “the addition of AS significantly reduced post-treatment prevalence and mean density of gametocytes in the first 14 days, although by day 28 the benefits of the combination were substantially less marked. The duration of gametocyte carriage over the study period was significantly lower in the CQ/AS group. The estimated infectious proportion of children at day 7 was also lower in the combination group as were the proportion of mosquitoes infected and mean oocyst density”49 and therefore “the benefits of adding AS to CQ monotherapy in lowering gametocyte prevalence and density were transient, suggesting that the addition of AS delayed but not prevent the emergence of gametocytes”.49
In Mali, following artemether-lumefantrine treatment, gametocyte carriage decreased steadily from Day 0 to Day 21 post-treatment initiation.50 In contrast, for the artesunate-amodiaquine and artesunate-sulfadoxine/pyrimethamine arms, gametocyte carriage increased on Day 3 and remained constant until Day 7 before decreasing afterward. Mosquito feeding assays showed that artemether-lumefantrine and artesunate-amodiaquine significantly increased gametocyte infectivity to Anopheles gambiae sensu lato whereas artesunate-sulfadoxine/pyrimethamine decreased gametocyte infectivity in this setting (p=0.03).50
According to such diversity of antimalarial effects, including some well-known risks, it was interesting to see whether vector control operations, with conventional deltamethrin insecticide treated nets (LLIN) or lambdacyhalothrin Inside Residual Spraying (IRS) or more modern tools (deltamethrin treated durable lining alone or associated with LLIN or following IRS), could have an effect on gametocyte index and at which level.
The microscopical observation and analysis of the 23,822 thick blood smears (TBS) of children ≤15 years old prepared during the 234 parasitological cross-sectional surveys done for the long-term malaria vector control program carried out in 8 villages of the Balombo region (Benguela Province, Angola) since 2007, showed a significant impact of the vector control not only on the plasmodic index22 but also on the gametocyte index. This impact was observed at short, middle and long term, from an initial values of about 4% quickly decreasing by half after vector control implementation and remaining at 0.5% for 10 years later, even with some years without microscopically detected gametocytes, and a comparable efficacy regardless of the control method used.
Such observation is of great epidemiological and operational importance for the Plan of Action (PoA) of the National Malaria Control Program.
Actually, a cross-sectional survey carried out in 2015 in a nearby village, not involved in our study, reported a gametocyte index of 4.5% in children ≤15 years old (PC. unpub. obs.), similar to what was noticed at the beginning of our trial, while it was kept very low at the same time, with a value of 0.2% in the project villages, treated 7 years earlier, showing that the effects observed were not simply due to an ecological or any other factor, but one of the impacts of the vector control operations. Even with the onset of a malaria outbreak at the national level, gametocyte index in treated villages remained lower than in the surrounding ones, although the trend of the populations to remove plastic sheeting or mosquito nets must be taken into consideration for the sustainability of the results obtained.
The fact that gametocyte index determined with Light Microscopy (LM) decreased after vector control (VC) implementation rises several questions about the actual efficacy of VC in reducing the infectivity of human population to anopheles vectors. For instance, new method such as the high field gradient magnetic fractionation (HFGMF), detect carriers with gametocytes densities lower than observed by the standard light microscopy but who could be infective for anopheles.51 For Karl et al,51 their analyses indicate that models which include only moderate-high gametocytaemia (detectable by LM) predict finite eradication times after LLIN introduction. Models that include a low gametocytaemia reservoir (requiring PCR or HFGMF detection) predict much more stable, persistent, transmission and their outcomes result in significantly different estimates for the level and duration of control needed to achieve malaria elimination if submicroscopic gametocytes are included and there is therefore a risk of premature termination of control measures followed by resurgence of disease. The fact that during several years we did not observed gametocytes among several thousands of blood smears could be attributed to the low level of gametocytaemia, may be lower than the detectability threshold of our LM classical technique. For the targeted malaria elimination it should be recommended the local development of new tools/methods/techniques to improve the diagnosis and monitor the efficacy of vector control operations implemented when considering that with classical Light Optical microscopy (LM) the observation of one gametocyte while counting 200 leucocytes means a detectability of 40 sexual elements/ml of blood; while new molecular methods allow some limit of detection (LoD) of 0.02-0.05 gametocytes/ml of blood.52
For example in Papua New Guinea, vector control has been intensified since 2008.53,54 Cross-sectional surveys were conducted in 2006, 2010 and 2014; infections were quantified by highly sensitive quantitative polymerase chain reaction (PCR) analysis, and gametocytes were quantified by reverse transcription qPCR (rt-qPCR) analysis, which greatly improved the detectability of gametocytes compared to classical LM.54 These surveys showed that P. falciparum gametocyte prevalence decreased 3-fold from 2010 to 2014; the majority of gametocyte carriers determined by rt-qPCR were LM positive for asexual parasites in 2010, but in 2014 approximately two thirds of gametocyte carriers presented submicroscopic infections. This study showed that asymptomatic and submicroscopic infections carry gametocytes that were infective to mosquitoes.53 Therefore, it was considered that sustained control resulted in reduced malaria transmission potential, but an increasing proportion of gametocyte carriers were asymptomatic and submicroscopic representing a challenge to malaria control.
In another study using membrane feeding experiments in Burkina Faso,55 Ouedraogo et al.55 also underlined the substantial contribution of submicroscopic P. falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. They reported that children with microscopically detectable gametocytes were more likely to be infectious (68.2% compared to 31.7% of carriers of submicroscopic gametocytes) and on average infected more mosquitoes (13.2% compared to 2.3%). However, because of the high prevalence of submicroscopic gametocyte carriage in the study population, carriers of sub-microscopical gametocytes were responsible for 24.2% of the malaria transmission in this population.55
The increasing number of gametocyte carriers revealed by new biological analysis method, and not by the classical LM, added to the infectivity of even low-density gametocytes carriers, underlined the need of strengthening National capacities with new strategies for detection of Plasmodium gametocytes.52 But even with the currently available tools, some great impact on malaria transmission can be obtained with sustained comprehensive vector control. The required long-lasting duration of vector control underlined in these studies has to be considered with the long-term impact on gametocyte index obtained by the different methods of vector control implemented in the Balombo project and the human behavior in term of removing or using regularly the nets.
The long-term (11 years) monitoring of gametocyte index in villages where vector control operations were implemented (with 4 methods) procured 2 important information for the National Malaria Control Program (NMCP). According to the classical light microscopical (LM) method, vector control reduced by about 80% the human reservoir of Plasmodium infective stage for vectors and this reduction was observed during 4 consecutive years in our trial. On the other hand, it is possible that vector control reduced the density of gametocytes below the threshold of LM detectability and new molecular methods should be usefully developed for a better evaluation of large-scale vector control program. It should also be interesting to develop such studies in other epidemiological facies, at National and Regional levels, and with other Anopheles vector species.
None.
The author declares there is no conflict of interest.

©2022 Carnevale, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.