MOJ
eISSN: 2379-6383


Research Article Volume 8 Issue 4
1Molecular Diagnostics, Apollo Hospitals Dhaka, Bangladesh
2Dermatology & Laser Center, Apollo Hospitals Dhaka, Bangladesh
Correspondence: Mizanur Rahman, Molecular Diagnostics, Apollo Hospitals Dhaka, Bangladesh, Tel 8801755646545
Received: April 30, 2019 | Published: July 12, 2019
Citation: Rahman M, Rahim R, Hasan A, et al. Distribution of human papillomavirus genotypes in genital warts in patients attended a tertiary care hospital in Dhaka, Bangladesh. MOJ Public Health. 2019;8(4):124-128. DOI: 10.15406/mojph.2019.08.00296
Introduction: Human papillomavirus (HPV) association in genital wart is well known, however, data are mostly from developed countries and no data from Bangladesh is yet available.
Methods: In order to see the HPV association and type distribution in genital warts in Bangladeshi patients, we screened DNA from warts and checked presence of HPV by real time polymerase chain reaction (PCR).
Results: Out of 44 wart specimens from 44 patients, 30(68.18%) were found positive for HPV. Out of these 30 positive patients low risk HPVs were 25 (83.33%) and high risk HPVs were 3(10%) and co-infection with low risk and high risk HPVs were 2(6.66%). Among the low risk HPVs, type 6 was found in 23(85.18%) and type 11 was found in 4(14.8%) indicating high dominance of HPV type 6. Among the high risk HPVs, type 16 was found in one, type 18 was found in one and HPV other than type 16 and 18 was found in three patients. Though the number of male patients in this study was smaller than female patients (10 vs 34) type distribution of HPVs in warts from male and female are similar.
Conclusion: Although it is accepted that HPV 6 and 11 genotypes are main causes of warts, our findings show non-negligible incidence of multiple infections and high-risk genotypes in both male and female with benign HPV manifestations (warts). This is the first report of HPV documentation and type distribution in genital warts in Bangladesh and hence demand further large scale study.
Keywords human papillomavirus, HPV genotypes, genitals warts, bangladesh
According to Cartier’s definition1 genital wart is defined as flesh-colored, exophytic lesions (small bumps, flat, verrucous, pedunculated, raised papules or dome-shaped lesions on keratinized skin) on the external genitalia, including perineum, and perianal skin. The diagnosis of genital warts is based on visual inspection. They are one of the most common types of sexually transmitted infection. They are also known as venereal warts or condylomata acuminata which consist of fibrous overgrowths covered by a thickened, outer layer. They can appear around a woman's vulva, cervix, vagina, anus and a man's scrotum, anus, and penis. They are contagious, usually benign, or non-cancerous, but some types can become cancerous in time. In appearance, genital warts are often flesh-colored or gray swellings. If several cluster together, they may resemble a cauliflower. Sometimes, it may be too small to be seen by the naked eye. Human papillomavirus (HPV) infections are the causative agents of genital warts and many genital and oral cancers.2 HPV is one of the most frequent sexually transmitted viral infections and has more than 120 identified virus types.2–5 HPVs are divided into the high-risk group and the low-risk group according to the carcinogenicity or into the cutaneous type and the genital-mucosal type according to the tissue of development.2 The HPV genotypes common in genital wart include HPV-6, 11, 16, 18, 31, 33∼35, 39, 40, and 51∼60 and among them, HPV-6 and 11 belong to the low-risk group related to genital and cervical cancer.2 In most of the genital warts low risk, HPV 6 and 11 are found and in few percentages of cases, high-risk HPVs are found.2 However, these data are from developed countries and there is no data from Bangladesh regarding association of HPVs in genital warts.
Genital warts are highly infectious and nearly 65% of individuals with an infected partner develop lesions within 1 to 8months from the first contact.4,6 HPV prevalence varies by age and is more common for young men and women with a new sexual partner.7 Genital wart prevalence estimates by country range widely from 1.4% in Spain to 25.6% in Nigeria8,9 and 0.9% in Hong Kong, 0.7% in South Korea.10
Data on genital warts in South Asia is not available. A recent elaborated study from India showed genital wart prevalence in India is 1.07% and is higher among men than women11 there is neither any report of genital wart incidence and prevalence in Bangladeshi nor any study of HPV association in genital wart in Bangladeshi people. We studied, for the first time, the association of HPV in genital warts in 44 patients visited Apollo Hospitals Dhaka during 2015 to 2019.
Clinical specimens and ethical clearance
This study proposal was approved by the Research and Ethical Practices Committee of Apollo Hospitals Dhaka (approval number ERC 22/2018-4). Clinical specimens which included different site specific genital warts from 44 patients consisting 34 females and 10 males were collected at the dermatology outpatient department (OPD) and gynecology and obstetrics department by the dermatologist and gynecologist respectively, at the Apollo Hospitals Dhaka during a routine test for HPVs. Extracted DNA from samples were studied for both high risk and low risk HPVs. Further, genotyping of HPV 16 and HPV 18 were done on all HPV high risk positive samples. De-identified stored DNA from wart specimens were used for this study purpose.
DNA extraction
DNA extraction was done with DNA-Sorb-A (Sacace, Italy, K-1-1/A). Specimen was collected in falcon tube containing transport medium provided in the kit (Sacace, Italy). The warts were chopped with a scalpel in a petri dish and transferred into a micro centrifuge tube containing PBS. Then the tube was centrifuged and supernatant was discarded. Cell pellet with the 100ul of residuals was lysed by 300ul lysis buffer (Sacace, Italy) for 5minute at 65°Cand then centrifuged. Supernatant was discarded and then Sorbent was added and mixed well and then incubated at room temperature for 3minute. After brief centrifuge pellet was washed twice with washing solution. Finally, DNA-elute (Sacace, Italy) was added to pellet and incubated for 65°Cfor 5minute and centrifuged and DNA in the supernatant was collected in a new micro centrifuge tube and stored at -80°Cif not amplification is done in the same day of extraction. One HPV negative sample provided by the kit producer was also extracted in similar way at the same time of clinical sample processing.
HPV detection
We used CE-IVD marked real time PCR kit (Sacace, Italy) for HPV detection and typing. 10ul of extracted DNA was used in PCR reaction for high risk, HPV 16/18 typing and low risk. Target region of amplification for high risk HPV is E1 and E2 region. The primer sequences are not disclosed by the kit producer.
HPV high-risk real time PCR
HPV High Risk Screen kit (V31-100/2FRT) is an in vitro Real Time amplification test for the detection of HPV (type 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59). 10ul extracted DNA was mixed with 15ul of PCR reaction Mix composed of 7ul of PCR-mix-1-FRT and 8ul of PCR-buffer-FRT/TaqF DNA polymerase. PCR-mix-1 tube contains primers directed against regions of HPV A7, A9 groups (HPV types 16, 18, 31, 33, 35, 39, 45, 52, 58, 59), HPV A5 group (HPV type 51), HPV A6 group (HPV type 56) and b-globin gene used as Internal Control. Reagents were mixed by tapping the tubes and transferred the tubes in the PCR machine (Rotor Gene Q, Qiagen, Germany) along with positive control DNA (10ul provided in the kit) tube and processed negative control tube. PCR protocol was programmed according to kit manufacturer’s instruction. In brief, a hold of 15minutes at 95°Cwas followed by first fluorescence non-acquiring 5cycles (95°C 5seconds, 60°C 20seconds, 72°C 15seconds) and then fluorescence acquiring 40cycles (95°C 5seconds, 60°C 20seconds, 72°C 15seconds). Threshold was set manually and analysis was done according to instruction. The result of the sample was considered positive if in the Joe (yellow) channel the fluorescence signal is present (Ct≤ 33) at least in one of the 2 tubes. In case of positive samples fluorescence signal in the Fam channel may be absent. Sample was considered negative if amplification was found only in the Fam (green) channel.
HPV 16/18 typing
HPV high-risk positive samples were further checked for presence or absence of HPV 16, 18 by HPV 16/18 Real time PCR kit (Sacace, Italy). Amplification results of HPV 16 DNA were detected on the Green channel, amplification results of HPV 18 DNA were detected on the Orange channel and β-globin gene used as Internal Control was detected on the Yellow channel. PCR protocol was same as HPV high risk screening PCR.
HPV low-risk (6, 11) real time PCR
10ul extracted DNA was mixed with 15ul of PCR reaction mix composed of 10ul of PCR-mix-1-FRT and 5ul of PCR-buffer-FRT/TaqF DNA polymerase. PCR-mix-1 tube contains primers directed against L1 gene of HPV types 6, 11 and b-globin gene was used as Internal Control. If the tissue/swab is not correctly prepared (insufficient quantity of epithelial cells) the Internal Control will not be detected or will be low (the quantity of epithelial cells lower than 103cells/reaction). HPV 6 was detected on the Green channel, HPV 11 on the Yellow channel and Human b-globin gene on the Orange. PCR protocol was same as it is mentioned above for HPV high risk amplification.
We evaluated HPV association in genital warts of 44 patients that visited our dermatology and gynecology departments during 2015 to 2019. Out of these 44 patients, 34 were female and 10 were male patients. Among female patients 2 were children and 32 were adults. Age ranges of female and male adults were 25 to 58 and 25 to 68years respectively. Age group of study populations (44 patients) and number of HPV positive cases with percentage among each group are shown in table. Four adults and two children were in the age range of less than 25 years old. Warts from these 2 children were HPV negative. Three of the 4 adults were females and warts from them were HPV positive, while wart from the male adult was HPV negative. Though high percentages of HPV positive warts were observed in the upper age groups, the highest percentage of HPV positive warts were observed in the 40-49 age groups. HPV positive cases decreased after the age of 50 (Table 1).
Age in years |
No. of cases |
HPV+ no. (%) |
<25 |
6 |
3(50%) |
25-29 |
9 |
5(55.5%) |
30-34 |
6 |
4(66.6%) |
35-39 |
9 |
7(77.7%) |
40-44 |
3 |
3(100%) |
45-49 |
3 |
3(100%) |
50-54 |
3 |
1(33.35) |
>55 |
5 |
4(80%) |
Total |
44 |
30(68.18%) |
Table 1 Age distribution of total and HPV+ cases
Out of 44 wart specimens from 44 patients, 30 (68.18%) were positive for HPVs (Figure 1). Among the 30 HPV positive cases, 22 were female and 8 were male patients. Of these 30 HPV positive warts, 27 were of HPV low risks type, and 5 were of HPV high risks type that included 2 co-infections for the both types. Out of these 27 low risk HPVs, 23 were HPV type 6, and 4 were HPV type 11 (Figure 1).
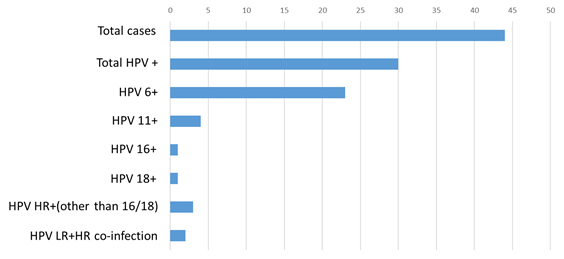
Figure 1 Total distribution of HPVs in warts among total (both gender) study population.
HPV, human papilloma virus; HR, high risk; LR-low risk
Distribution of HPVs in wart in female patients
Wart was primarily diagnosed on visual examination by the attending dermatologist or gynecologist at their respective clinic. A small clip of tissue and/or swab is taken by the physician at their clinic and sent to molecular lab for HPV confirmation by PCR. We extracted DNA from wart specimens from 34 females who attended our dermatology and gynecology clinic. Out of the 34 females, we found 22 patients (64.7%) were HPV positive (Figure 2). Among these 22 positive cases, 19 (86.36%) were low risk HPVs and 2 (9.09%) are high risk HPVs and one co-infection with high risk and low risk HPV (Figure 2). Among the 20 (including one co-infection) low risk positive cases, 17 (85%) were HPV 6 and 3 (15%) were HPV 11. Among the two high risks positive cases one was HPV 18 and one was HPV other than 16/18. Interestingly, one specimen was found positive both for low risk (HPV 6) and high risk (HPV other than 16/18).
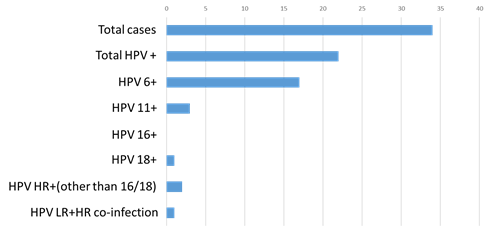
Figure 2 Distribution of HPVs in warts in female.
HPV, human papilloma virus; HR, high risk; LR-low risk
Distribution of HPVs in wart in male patients
We extracted DNA from wart specimens from 10 male patients who attended our dermatology clinic. Out of 10, we found 8 patients (80%) were HPV positive (Figure 3). Among these 8 positive cases, 7 (87.5%) were low risk HPVs and one (10%) was high risk HPV and one co-infection with high risk (HPV 16) and low risk (HPV 6). Among the 7 low risk positive cases, 6 (85.7%) were HPV 6 and 1 was HPV 11 (Figure 3). Among the two high risks positive cases, one is HPV 16 and one is HPV high risks other than 16/18. Similar to female group, one case is found both positive for low risk (HPV 6) and high risk (HPV other than 16/18).
In this study we show evidence of association of HPVs (68.18%) in genital wart in Bangladeshi patients. High (over 87%) association of low risk HPVs are found both in male and female patients. Among the low risk HPVs, high (85%) dominance of HPV 6 is found. Further, we have seen the association of high risk HPVs and co-infection with low risk HPV and high risk HPV in both male and female patients within this small number of study population.
Genital infection with HPV is one of the most common sexually transmitted diseases (STDs) of viral etiology worldwide. Genital warts are highly infectious and in order to stop the spread of infection, apart from education about precautions, early detection and treatment of the disease is essential. Although most sexually transmitted pathogens can be detected by classical methods of cultivation, biochemical and/or serologic methods, molecular diagnosis of infectious diseases has largely simplified and accelerated their detection. For instance, HPVs that cause benign and malignant tumors of genital skin and mucosa cannot be routinely detected on cell culture, whereas serologic analysis is not sensitive and informative enough. That is why the molecular methods are essential to demonstrate the presence of the infection and more important, to determine the type of the virus, which is associated with low-grade or high-grade genital lesions.
Genital wart is known to be difficult to differentiate from lesions such as nevus, benign keratosis, cyst, an ectopic sebaceous gland, and syphilitic condyloma,12 the key point for differentiating wart from these diseases is detecting HPV in the lesion, and the most sensitive method for proving HPV is PCR. Where PCR is not available immunohistochemistry can be used as an alternative method.13
Data on genital wart in South Asia is sparsely available. Overall genital wart prevalence in India was estimated recently at 1.07% and was higher among men than women.11 Consistently higher prevalence of HPV in men than women is found which is explained by strong immune response in women as supported by serologic studies.14,15 A higher prevalence of HPV antibodies in women than men across all ages were shown in these studies.14,15 Although our study is not a prevalence study, higher percentages of HPVs are found in warts in males (87.5%) than in women (65.5%). We cannot exclude the possibility that HPV negative specimens may have HPVs other than those of our targeted ones.
Much of the information about HPV centers on women, since having the virus increases their risk of cervical cancer. HPV prevalence in cervical swab of normal cervical cytology in India is 19.11% and 7.7% in Bangladesh.16,17 There is neither any study of prevalence of genital wart in Bangladesh nor any report of HPV association with genital wart. Although genital warts are benign and not associated with mortality, they are a source of psychosocial distress18 and can cause physical discomfort, including pain, bleeding, and itching.19 Genital warts are highly infectious; 65% of persons who have sex with a partner with genital wart will develop genital wart.20 A high rate of recurrence makes treatment difficult and costly.21
Genital warts may cause a significant burden in the community in regard to cost and psychological stress. Physical examination and HPV confirmation is recommended for early detection and treatment of HPV infection to reduce transmission. Further, prevention with a quadrivalent HPV vaccine protecting against HPV 6, 11, 16, 18 may be a promising preventive strategy.
The limitation of this study is small number of patients. Large scale multi-center based study is needed to know the exact HPV association in genital warts in the country.
Although it is accepted that HPV 6 and 11 genotypes are main causes of warts, our findings show non-negligible incidence of multiple infections and high-risk genotypes in both male and female with benign HPV manifestations (warts). This is the first report of HPV documentation and type distribution in genital warts in Bangladesh and hence demand further large scale study.
None.
Author declares there are no conflicts of interest.

©2019 Rahman, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.