MOJ
eISSN: 2379-6383


Research Article Volume 6 Issue 5
1Department of Water Pollution Research, National Research Center, Egypt
2Department of Plant and Microbiology, Al-Azhar University, Egypt
3Center of Scientific Excellence for Influenza Viruses, National Research Centre, Egypt
Correspondence: Mohamed Shaheen, Environmental Virology Laboratory, Department of Water Pollution Research, Environmental Research Division, National Research Center, Al-Buhouth Street 12622 Dokki, Giza, Egypt, Tel 002 01016710071
Received: October 25, 2017 | Published: December 5, 2017
Citation: Shaheen M, Abd EDSE, Hosseney EN, et al. Molecular characterization of rotavirus strains causing gastroenteritis in children under 5years in Cairo, Egypt. MOJ Public Health . 2017;6(5):428-432. DOI: 10.15406/mojph.2017.06.00187
Background: Rotavirus infection has been identified as the most common pathogen associated with acute gastroenteritis in infants and children worldwide.
Objective: This work was designed to study the occurrence of rotavirus among children less than 5 years with acute diarrhea admitted to Abu-El Rish hospital in Cairo, Egypt.
Methods: 198 stool specimens were collected during the period from May 2015 to April 2016. The samples were tested for rotavirus by enzyme immunoassays (EIA) them rotavirus-positive specimens were G-genotyped by the semi-nested multiplex reverse transcription polymerase chain reaction (RT-PCR) using different type specific primers.
Results: Out of 198 collected samples rotavirus infection was detected in 56 (28.3%). Of the rotavirus diarrhea, 64.3% occurred during the first year of life, with the peak prevalence of severe rotavirus disease in March and April. Among the common genotypes, G3 was the most predominant (17.8% of strains). Other identified genotypes such as G1, G9, and G10 were detected separately in 5.3% of the positive samples, whereas G4 was detected in only 1.8%. Furthermore, G2 was not found in this study. The most circulating mixed G types were G1+G3+G8 (17.8%), followed by G3+G9 (1.8%).
Conclusion: The current study demonstrated that rotavirus accounted for 28.3% of gastroenteritis, particularly among children under 1 years of age with a high prevalence of the G3 genotype. Our findings provide useful data for future vaccine development approaches in Egypt.
Keywords: Rotavirus; Gastroenteritis; Children; Vaccine
Rotavirus; ELISA: Enzyme-linked immunosorbent assay
Globally, human rotavirus (RV) remains the most common cause of severe diarrhea in children, causing 453,000 deaths per year and proximately 2.4 million hospitalization among children aged less than 5 years, with a maximum incidence in the developing countries [1-3]. Transmissions of RVs occur mainly via fecal-oral contact but it also might be transmitted by respiratory spread [4,5]. RV group A is the most cause of viral gastroenteritis in human and at least 27 G-serotypes and 32 P-serotypes have been identified [6,7]. Among them, 12 G-serotypes (G1 to G6, G8 to G12, G22) and 15 P-serotypes (P[1] to P[11], P[14], P[19], P[25]) have been identified in human [8-11]. Globally, G1 to G4 and recently G9 are the major G-serotypes in human. Globally uncommon G-serotypes such as G5, G6, G8, and G10 to G12 may be detected regionally [9-11]. On the other hand, P[4], P[6] and P[8] are the most prevalent human P-serotypes [12,13], while rare human P-serotypes P[9], P[11] and P[14] are increasingly identified locally in different areas of the world [14,15]. Furthermore, novel RV serotypes can be produced from mixed infection of segmented RV genome. Two oral live attenuated vaccines, pentavalent RotaTeq (Merck and Co. Inc.), consisting of G1-G4 types with P[8], and monovalent Rotarix (GlaxoSmithKline), consisting of G1 with P[8], to prevent RV gastroenteritis are currently licensed in lower middle income countries [16] and European countries [17]. In this study, we studied the incidence of RV genotypes among children less than 5 years old circulating in Egypt to determine whether the current vaccines cover the most circulating RV among children below 5 years old in Egypt.
Collection and preparation of stool specimens
A total of 198 fecal specimens were collected from children less than 5 years old, who were hospitalized and non-hospitalized with diarrhea at the public Abu-El Rish hospital, Cairo, Egypt. Samples were collected and given special codes by the hospital laboratory team. So we don’t need ethical approval for this work. The study period was 1 year (from the first of May 2015 to the first of April 2016). Approximately 100 mg of each fecal specimen was initially suspended in 1 ml of phosphate-buffered saline (pH=7.0). After mixing and centrifugation at 4.500 rpm for 10 min at 4°C, the supernatant was transferred into new Eppendorf tube then stored in -80°C until examined.
Detection of rotavirus antigen by Enzyme-linked immunosorbent assay (ELISA)
ELISA method was used for detection of rotavirus antigens in the collected samples. RIDASCREEN® Rotavirus (R-Biopharm, Germany) kit with 100 µl of each sample were used to determine the presence of RV antigen. The microwell plates were coated with monoclonal antibodies against the middle layer antigen of RV (VP6) in a sandwich type method. The test procedure was performed according the manufacturer’s instruction [18]. The specimen was considered positive if the optical density (OD) was higher than the cut off value (OD of the negative control + 0.15). All specimens were tested in duplicate, and the positive samples for RV were then subjected to viral RNA extraction and semi-nested multiplex RT-PCR tests.
Viral RNA extraction
RV genome was extracted from 10% stool suspension of each specimen by QIAamp Viral RNA Mini Kit (Qiagen, Germany), according to the protocol described in the manufacturer’s guidelines.
Reverse transcription-polymerase chain reaction (RT-PCR)
cDNA was synthesized in a final volume 25µl: 5μl of the extracted RNA with 1µl of reverse primer VP7-R (50µg/µl) for G-types was heated at 65oC for 10 minutes and 4µl of 2.5mM dNTPs, 10µl of 5x RT buffer, 1µl of MMLV reverse transcriptase enzyme (10U/µl), and 4µl DEPC-treated water were added. To produce the complementary (cDNA), the total mixture was heated at 42°C for 1 h, followed by 37°C for 30 min then finally 95°C for 5 min to inhibit RT enzyme.
G-genotyping
Amplifying of a VP7 gene was carried osut using two steps according to protocol described previously by Iturriza Gomara [19]. Briefly, the first round was carried out in a final volume 50 µl consisting of 5 of cDNA mixed with 10 µl of M-MLV 5X reaction buffer, 4 µl of 25 mM MgCl2, 4 µl of 10 mM dNTPs, 0.25 µl of 5 U/ml Go Taq DNA polymerase (Promega, USA) and 1 µl of 25 pmol of each forward primer VP7-F and reverse primer VP7-R (Table 1), 24.75 µl DEPC-treated water. The PCR condition was carried out at 95°C for 5 min, followed by 35 cycles at 95°C for 1 min, 52°C for 1 min, 72°C for 1 min and then 72°C for 10 min as a final extension. The second round VP7 multiplex was prepared in 50 µl total volume containing a 2.5 µl of the first round product as a template was mixed with 1 µl of each G type-specific primer G1, G2, G3, G4, G8, G9 (Table 1), 10 µl of M-MLV 5X reaction buffer, 4 µl of 25 mM MgCl2, 4 µl of 10 mM dNTPs, 0.25 µl of 5 U/ml Go Taq DNA polymerase (Promega, USA), 23 µl DEPC-treated water. The total mixture was subjected to 95°C for 5 min followed by 30 cycles at 94°C for 1 min, 42°C for 2 min, 72°C for 1 min and then a final extension of 72°C for 10 min.
Primer |
Sequence (5-3) |
Product Size (bp) |
nt. Position |
References |
|
First Round |
VP7-F |
ATGTATGGTATTGAATATACCAC |
881 |
51-71 |
[20] |
VP7-R |
TGCCACCATTTTTTCC |
914-932 |
|||
Second Round |
G3 |
ACGAACTCAACACGAGAGG |
682 |
250-269 |
[19] |
G9 |
CTTGATGTGACTAYaAAATAC |
179 |
757-776 |
||
G10 |
ATGTCAGACTACARbATACTGG |
266 |
666-687 |
||
G8 |
GTCACACCATTTGTAAATTCG |
754 |
178-198 |
||
G4 |
CGTTTCTGGTGAGGAGTTG |
452 |
480-499 |
[21] |
|
G2 |
CAATGATATTAACACATTTTCTGTG |
521 |
411-435 |
||
G1 |
CAAGTACTCAAATCAATGATGG |
618 |
314-335 |
||
Table 1: Oligonucleotide primer for G typing (VP7) of rotavirus.
Nucleotide sequencing
The PCR amplicons of most common G-genotype were selected and purified with Qiagen purification kit (Qiagen, Germany), according to the manufacture's guidelines. Using the primers used in the amplification of VP7 in the first round, the purified amplicons were sequenced directly by automated DNA sequencer. The produced sequences were compared with available RV sequences in GenBank database.
Detection of rotavirus and its correlation to age and gender
Of the 198 samples tested, RV antigens were found in 56 (28.3%) samples. The highest rates of RV gastroenteritis were detected in male (35/56; 61.4%) than in female (21/56; 37.5%). RV gastroenteritis cases were occurred in all different age groups of children, but the majority of RV cases were observed in children aged less than 12 months, accounting for 64.3% of all rotavirus cases (Figure 1).
Seasonal distribution
As shown in Figure 2, RV gastroenteritis was occurred throughout the year, but the frequency of RV among patients was detected in April 2015 (10/56; 17.8%), followed by March 2016 (11/56; 19.6%), November 2015 (8/56; 14.3%). July 2015, December 2015, January 2016 had the same RV gastroenteritis cases with 6/56 (10.7 %). The lowest RVGE cases was found in September 2015 (2/56; 3.6 %), October 2015 (2/56; 3.6 %), and August 2015 (1/56; 1.8%). During the period from May to June 2015, RV gastroenteritis was not detected.
Rotavirus genotyping
G genotyping was performed on all RV positive samples (n=56) by using semi-nested multiplex RT-PCR (Figure 3). G3 was the most prevalent in the population (10/56; 17.8%), followed by G1 (3/56; 5.3%), G9 (3/56; 5.3%), and G10 (3/56; 5.3%), then G4 (1/56; 1.8). The most circulating mixed G types were G1+G3+G8 (10/56; 17.8%), followed by G3+G8 (1/56; 1.8). G1, G3, G9 types were observed separately and in mixed infection whereas G8 type was detected only in mixed infections. The genotype G2 was not found in this study.
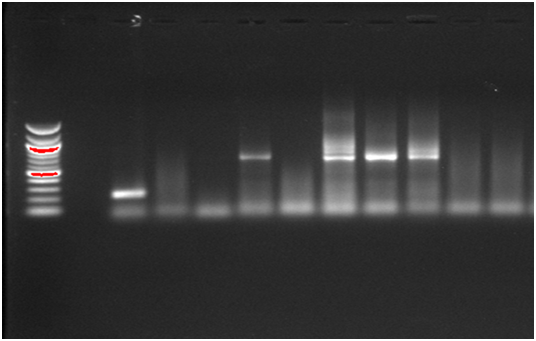
Figure 3: G types of rotavirus detected by semi-nested multiplex RT-PCR; Lanes 1: 12,000 bp molecular weight marker; Lane 2: Negative control; Lanes 3-12, samples: Lane 3: Genotype G10; Lane 6: Genotype G3; Lane 8: Mixed genotypes (G3+G8); Lane 9: Genotype G3; Lane 10: Mixed genotypes (G3+G8); Lanes 4,5,7, and 11 are negative RV.
Sequence and phylogenetic analysis
Nucleotide sequence of the Egyptian G3 isolate showed 99% nucleotide identity with rotavirus G3 isolate from Turkey and 98% nucleotide identities with rotavirus G3 isolates from Italy and China.
Rotavirus is the most common etiological agent of non-bacterial severe diarrhea in children, worldwide. This study was conducted to investigate the epidemiology of rotavirus infection among hospitalized and non-hospitalized children with acute diarrhea during 2015/2016 in greater Cairo. A total of 198 diarrheal specimens were tested by ELISA for rotavirus antigen and 56 (28.3%) were positive. This finding is within the range (11-76.9%) previously detected in Egypt [22-29] and in other countries (19-93.3%), including the Middle East and North Africa [30-40]. The variation in prevalence rates may be attributed to different conditions which may have affected the detection rates. For example, in other studies there were differences in the number of tested samples, season of sample collection, and the sampling methods. The occurrence of the group A Rotavirus was higher in the first 12 months of life (36%) than in the other age groups, as was observed in previous studies in developing countries [22-41]. This finding may be explained by decline of maternal antibodies with immature immune systems which protect the newborns from pathogens during the first months of life [42]. In the current research, of the 56 characterized G types, G3 was the most circulating rotavirus strain. This finding is similar to other previous results from Egypt and Tunisia during the periods August 2011 to August 2012 and June 2009 to 31 May 2011, respectively [29-43]. G3 genotype was also the most prevalent rotavirus genotype during the periods, August 2001 to July 2003 and June 2006 and February 2008 in China [44,45]. Otherwise, two studies from Egypt and Iraq demonstrated that G2 rotavirus was the most common rotavirus strain during the periods, March 2006 to February 2007 and January 2008 and December 2008, respectively [26-46]. However, G2 was not detected in the current survey, suggesting that G3 strains became the dominant during the latter period. In the present research, G1, G4, G8, G9, and G10 have been identified in 5.3%, 1.8%, 5.3%, 5.3% of all positive specimens, respectively. El-Senousy and El-Mahdy [47], reported that G1 was the most circulating genotype in the Egyptian environment during the period, October 2006 to September 2007. Other epidemiological studies from the Middle East and North Africa, including Tunisia, Saudi Arabia, Iraq, Morocco, Turkey, Libya, and Iran have reported that G1, G9 are the most common rotavirus G types [30-55]. Also, in the present epidemiological study, the genotype G8 was not observed individually but in mixed infections with G1/G3. Notably, the multiple infections of G1/G3/G8 and G3/G8 are reported for the first time in Egypt. Mixed infections with more than rotavirus strain might implies frequent contamination of water bodies with rotavirus strains, and could promote generation of new rotavirus serotypes through reassortment. Therefore, the efficacy of rotavirus vaccines should be continually investigated.
Our data suggest that the rotavirus still the important cause of gastroenteritis among children less than 5 years of age. Moreover, RotaTeq vaccine containing G3 rotavirus is more suitable for the current status in Egypt to prevent rotavirus infection in infants and children.
None.
None.

©2017 Shaheen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.