MOJ
eISSN: 2373-4442


Mini Review Volume 3 Issue 6
Program in Cell Biology, PGCRL, The Hospital for Sick Children, Canada
Correspondence: Yadu Balachandran, Program in Cell Biology, Cell and Molecular Therapies Division, Peter Gilgan Centre for Research and Learning (PGCRL), The Hospital for Sick Children, Toronto, M5G 0A4, Canada, , Tel 416-813-7654, Fax 416-813-5028
Received: August 11, 2016 | Published: September 1, 2016
Citation: Balachandran Y (2016) Role of Endocytosis in TLR Signaling: An Effective Negative Regulation to Control Inflammation. MOJ Immunol 3(6): 00111. DOI: 10.15406/moji.2016.03.00111
Cells take materials from outside without crossing plasma membrane through engulfing them by a process called endocytosis. It can be categorized into phagocytosis, pinocytosis, clathrin and caveolins mediated endocytosis. Toll-like receptor 4 (TLR4) is an innate immune receptor recognizing specific microbial pattern and initiate host response against invading pathogen. Receptor mediated endocytosis (RME) involve in the uptake of ligand activated TLR4 and helps to down regulate the proinflammatory pathway to prevent from septic shock, multiple organ failure and death. Endocytosed TLR4 can activate TIRF-TRAM pathway and produce IRF3 as effector molecule. Later in lysosomes, the endocytosed TLR4 undergoes Triad3A mediated degradation and thereby limiting its further signaling. This review discusses the latest findings on the role of endocytosis and receptor trafficking in TLR4 signaling.
Keywords: endocytosis; toll-like receptors; clathrin; caveolins; ubiquitination
TLR4: toll-like receptor 4; RME: receptor mediated endocytosis; CME: clathrin Mediated endocytosis; TGF-β: transforming growth factor β
Endocytosis is a process through which cells taking molecules in to the cell by bypassing plasma membrane trafficking. The physical and chemical properties of the molecule direct the cell to choose the method of internalization.1 After endocytosis, they form an independent vesicle in the cytoplasm known as phagosomes, pinosomes, coated vesicles etc. depending on the process of endocytosis. Classically endocytosis can be categorized in to four as phagocytosis, pinocytosis, clathrin mediated endocytosis (CME) and caveolae mediated endocytosis.2 Phagocytosis is a process of internalizing the particulate substrate, which varying in size from 0.05μm to 1.0μm. In pinocytosis, cells engulf lower molecular weight molecules and fluids. Pinocytosis can be further divided into two categories as adsorptive pinocytosis and fluid phase pinocytosis.3 The mechanism of endocytosis carried out through clathrin coated vesicles is meant for the internalization of antibodies, growth factors, and low-density lipoprotein. Caveolins form small conical invaginations in the plasma membrane, which will uptake the molecules through specific receptor mediated mechanism. Caveolins mediated endocytosis otherwise known as lipid raft /caveolar endocytosis since it carried out through lipid raft regions in most of the times.4–6
Both clathrin dependent and independent endocytosis play a vital role in cell signaling. A cholesterol-sphingolipids rich region on the plasma membrane known as lipid raft plays significant role in this mechanism. Cell membrane receptors are concentrated in the lipid raft and these lipid rafts are responsible for signaling molecule assembly and receptor endocytosis. Briefly, membrane proteins categorized in to three groups: ion channel linked receptors, enzyme linked receptors and G protein coupled receptors. Most of the receptors need to form a homo or hetero dimer in order to activate the downstream signaling cascade.7,8
Upon activation with specific ligand, the receptor recruits their primary adaptor molecules and triggers the activation of effector molecules. These receptors have extracellular, transmembrane and intracellular domains with functional importance. Some receptors endocytosed to the cytoplasm by choosing one of the above endocytic mechanism and in turn change fate of the signaling pathway.6 Guglielmo and colleagues explained the fate of Transforming Growth Factor β (TGF-β) receptor when it was endocytosed by CME and RME. During the CME, TGF-β activates the smad dependent signaling pathway whereas in RME mediated endocytosis leads to the degradation of TGF- β receptor.9 Chen and Camilla have shown the dose dependent endocytosis of epidermal growth factor receptor (EGFR). EGFR will choose CME if there is low ligand concentration and tend to undergo RME in the presence of higher dosage.10 The process of endocytosis regulates the signaling cascade and nutritional uptake of a cell.
Mechanism of receptor mediated endocytosis
Receptor mediated endocytosis is mainly mediated by clathrin. Adaptor proteins like AP-2 binds directly to clathrin and produce a ‘clathrin coat’. The ligand activation of a receptor will result in the binding of activator protein that in turn recruits the clathrin and they form a coated vesicle at the site of activation. Figure 1 explains the mechanism of RME. The vesicle fused with peripheral early endosomes in which the cargo may get processed inside. This fusion involves Rab5 and EEA-1 proteins. It is not clear that how and when the early endosomes transfer the cargo to late endosomes or the former matures to form later, even though they have distinct pH and chemical properties. The fate of the protein inside the endosomes, whether to recycle or degraded is determined by the chemical modification of the protein.11–16 Actin plays an important role in this process and Kaksonen et al have characterized the actin dynamics, movement and disappearance at the site of endocytosis.17 On the other hand, Dynamin-1 protein has no significance in the formation and recycling of endosomes.18,19
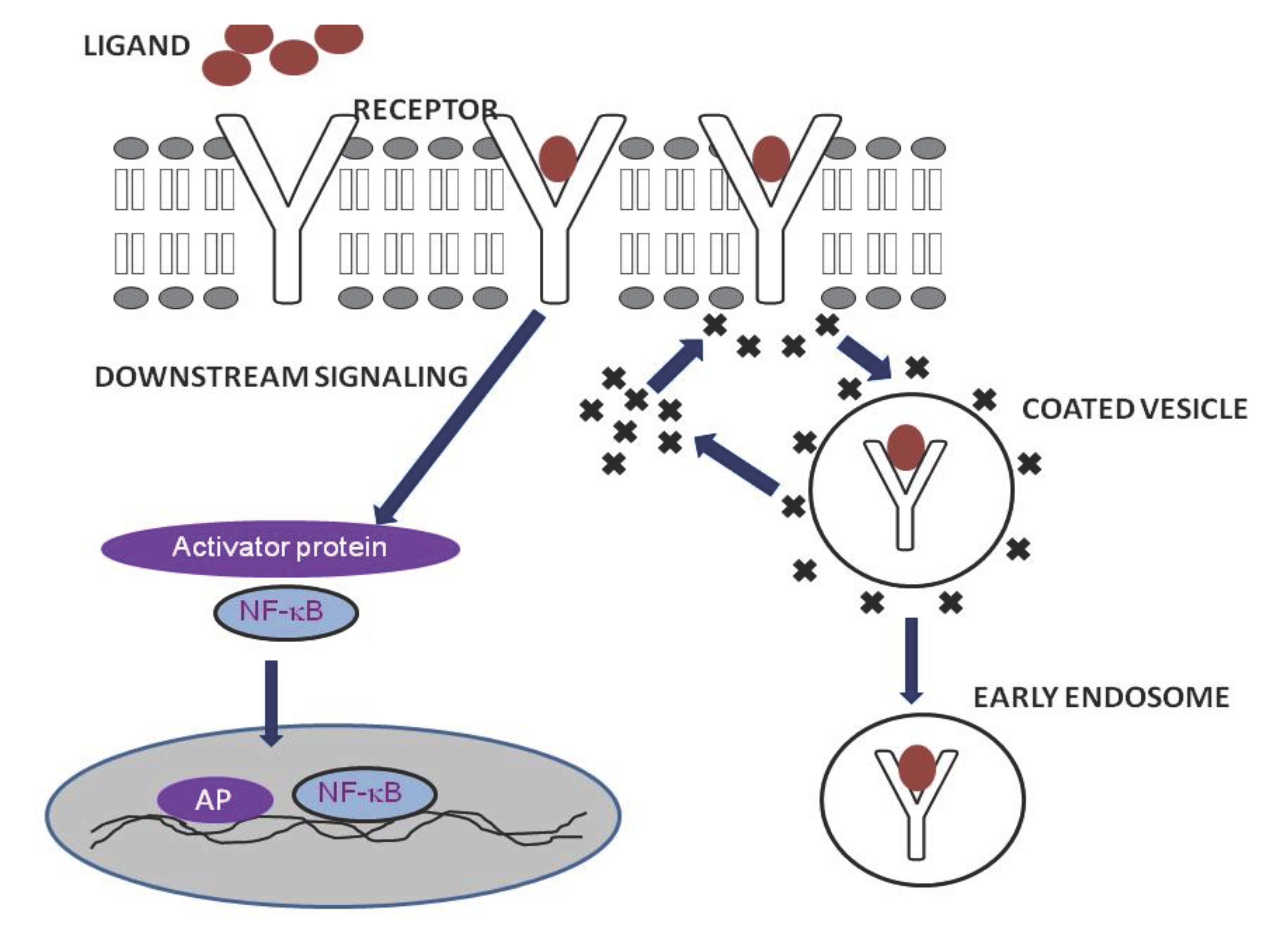
Figure 1 Process of receptor mediated endocytosis.
Receptor-mediated endocytosis (RME) is triggered by the binding of the specific ligands to host receptors. The ligand attachment to the host receptor induces the binding of an adaptor protein to the receptor cytoplasmic tail. Adaptor proteins bind to clathrin, and the local concentration of adaptor proteins on the inside face of the plasma membrane allows clathrin to multimerize to form characteristic invaginations or Clathrin-Coated Pit. Membrane scission proteins DNM1/Dynamin-1 or DNM2/Dynamin-2 pinch off the CCP from the host membrane thereby releasing the Clathrin-Coated Vesicle.
Endocytosis and regulation of signaling
Receptor activation through ligend binding requires additional adaptor molecules and phosphorylation to carry out the downstream signaling pathway. Endocytosis of the receptors leads to the down regulation of the signaling pathway, and ubiquity mediated degradation will take place in lysosomes or recycle back to plasma membrane from early endosomes. This helps the cell to retain the particular receptor activity simultaneously.15,20
A well-studied model for RME is EGF receptor endocytosis. Immediately after binding with EGF, the receptor localizes out of the raft area, eventually localized in to clathrin coat, and internalized. In the early endosomes, EGFR interacts with YOTB, Vac1, and EEA1 and forms a ternary protein complex with signal transduction adaptor molecule and EPS15. EGFR recycles back to the plasma membrane or transferred to multi-vesicular bodies for degradation.21-23 This type of internalization is meant to be a down regulation process for most receptor signaling. Kirchhausen and colleagues have shown the negative regulation of RME in EGFR.24
Toll-like receptor signaling and endocytosis
Toll like receptors are the ‘gate keepers’ of the innate immune system. They are responsible for the recognition and response towards invading pathogens.25,26 There are 13 mammalian TLR family proteins were identified so far. TLRs can be activated by variety of pathogen associated molecular patterns such as LTA, LPS, flagellin, CpG DNA, dsRNA, and ssRNA.27–31 Upon activation with the ligand, TLRs form homo or heterodimers and recruits its primary adaptor molecules, which will result in a series of cellular and molecular changes inside the cell. TLR signaling pathways follow two types of downstream activation namely, MyD88 dependent and MyD88 independent pathways, which eventually switch on the pro-inflammatory cytokine response through the nuclear translocation of NF-κB and IFN.25,32 TLRs which are present on the plasma membrane gradually endocytosed and it is found as an important negative regulation in TLR signaling as observed in EGFR previously.
TLR4 recognizes LPS and induces pro-inflammatory pathway via MyD88 mediated activation and nuclear translocation of NF-κB. Another way of TLR4 activation through TRIF-TRAM is introduced during its endocytosis. Continuous exposure to LPS may lead to septic shock and death of host cells, thus the host response should control the process of inflammation through ‘tolerance’. Endocytosis of the receptor and its degradation through ubiquity pathway is one of the negative regulatory mechanisms in LPS induced TLR4 pathway.33 TRAM is the signaling molecule for TLR4 translocation to endosomes with its bipartite signaling sequence. Kagan et al have explained the difference in host response of membrane localized and endocytosed TLR4 in macrophages.34 Haselby et al have shown the internalization of TLR4 within 30 min of LPS treatment and a GTPase known as dynamic regulates the process of endocytosis.33 LPS-CD14-TLR4-MD2 complex is getting internalized to make macrophage desensitized.25,33 Lee et al.35 showed the host tolerance of LPS on TLR4 signaling by using TLR4-GFP construct.35 Suri and colleagues previously reported the presence of TLR4 in the nucleus after stimulating with LPS.36 The mechanisms and functional aspects of this translocation yet to be explained.
Cellular and functional effects of TLR4 endocytosis
TLR4 is found to activate both MyD88 dependent and independent pathways. But no TLR can activate the MyD88 independent TRIF-TRAM signaling from plasma membrane. This mystery explained after the discovery of TLR4 localization into the endosomes. Internalized TLR4 activates a different pathway in early endosomes and purpose of this internalization is to limit the receptor expression (Figure 2). TLR4 signaling from plasma membrane activates the MyD88 adaptor molecule and leads to the activation of NF- κB and AP-1. These transcription factors are known to produce various pro-inflammatory cytokines.37 To avoid septic shock, multiple organ failure and death, the host will turn on ‘tolerance’ pathway by internalizing the TLR4 and limiting the MyD88 dependent pathway.3
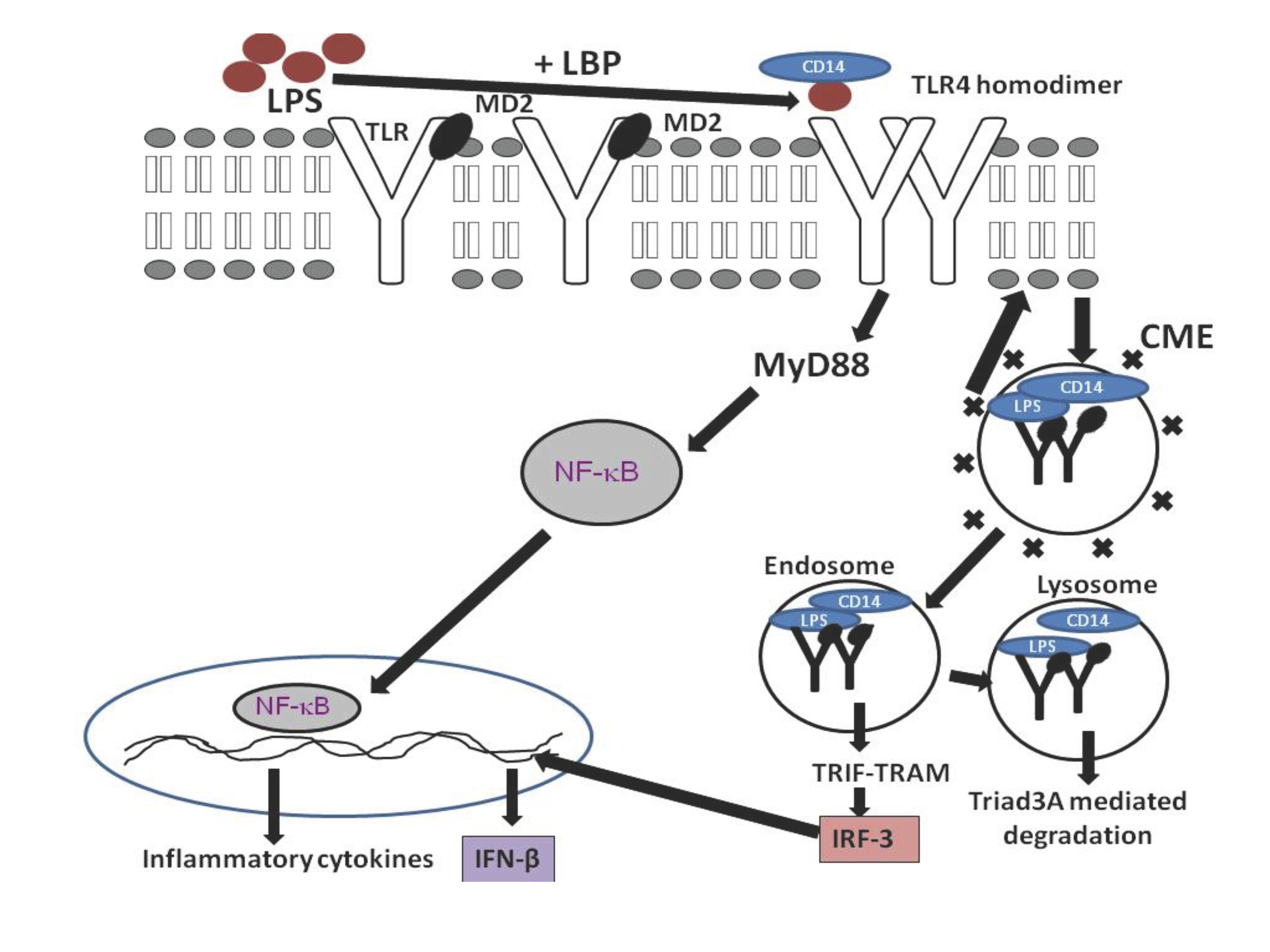
Figure 2 Mechanism of TLR4 signaling and endocytosis.
LR4 is found on the cell membrane and reacts to extracellular stimulation with lipopolysaccharide (LPS) with the help of LPS binding protein, CD14 and MD2. TLR4 signalling is terminated by endocytosis, ubiquitylation and lysosomal degradation, a mechanism that is likely to be shared by all TLRs.
TRAM mutant macrophages were unable to produce IL-6 and RANTES after stimulating with LPS. IRF3 will be induced after the activation of TLR4 induced TRIF-TRAM pathway from endosomes. The primary response genes for TRIF-TRAM pathway activation are IP10, RANTES, IFN-β, ISG15, and IFIT1 [38]. Hiscottl et al have reported that the cell is producing antimicrobial compounds such as ions and IFN-β upon the activation of TRIF-TRAM pathway.39 Gene expression profiling studies have revealed that the long-term exposure to LPS will lead the production of IFN-β.38
The activation of interferon pathway leads to the production of IFN- β, IP10, ISG15 etc. and will result in the activation of JAK-STAT pathway.40 TLR4 endocytosis also helps in the coordination between innate and adaptive immune systems. Degraded antigen bound TLR4 presented by class II MHC receptor and T helper cells recognize them to activate adaptive immune response.37
TLR4 activates two different pathways in which TRIF-TRAM mediated signaling refers the negative regulatory. Endocytosis of the receptor and ubiquity mediated degradation regulate an important role in negative regulation of signaling. Transfer of endocytosed TLR4 to the lysosomes and its degradation conflicts with the data showing their nuclear translocation and this differential immune response still need explanation.
Author expresses immense thanks to Dr. Baljit Singh, Dr. Willian Trimble, and Dr. Stacy Anderson for their guidance, advices and directions throughout.
The Author declares no conflict of interest related to this work and did not receive any fund for this study.
None.

©2016 Balachandran. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.