MOJ
eISSN: 2574-8130


Research Article Volume 7 Issue 4
1Department of Nephrology, Hospital Santo António, Centro Hospitalar Universitário do Porto (CHU Porto), Portugal
2UMIB - Unit for Multidisciplinary Research in Biomedicine, University of Porto, Portugal
3ITR - Laboratory for Integrative and Translational Research in Population Health, Portugal
Correspondence: João Paulo Pimenta Fernandes, Department of Nephrology Hospital Santo António, Centro Hospitalar Universitário do Porto (CHUPorto), Porto, Portugal Largo do Prof. Abel Salazar Porto, 4099-001, Portugal
Received: December 22, 2022 | Published: December 30, 2022
Citation: Fernandes J, Dias B, Campos A, et al. Mortality in elderly hemodialysis patients, influence of frailty and comorbidity. MOJ Gerontol Ger. 2022;7(4):90-95. DOI: 10.15406/mojgg.2022.07.00299
Background: Mortality in end-stage kidney disease (ESKD) remains high, especially among the elderly with a higher burden of comorbidity and frailty. In this group, dialysis may not offer better survival compared to conservative management. Frailty defined by clinical frailty scale (CFS) and comorbidity by Charlson Comorbidity Index (CCI) are known independent predictors of mortality. Our aim was to compare which one had higher impact on early mortality in urgent-start hemodialysis patients.
Methods: We conducted a retrospective cohort study of patients aged 65 years and over, who started hemodialysis as their first renal replacement therapy (RRT) between January 2014 and December 2020. CFS and mCCI, at time of HD start, were used to evaluate, respectively, frailty and comorbid disease burden. The primary outcome was death in the first 6 months of RRT.
Results: During the study period 166 patients were included. The median age, at time of hemodialysis start, was 75 years ± 6.3 years. The mortality at 6 months was 19% (n=31). For both scales, the analysis of ROC curve, stablished the optimal cut-off to predict the event death at first 6 months as ≥ 5points. The performance of CFS was superior to the mCCI, in fact, the area under the curve was significantly higher in CFS (0.739) versus the mCCI (0.620). A CFS≥5 had a sensitivity/specificity of 94%/44% in prediction the primary outcome. On the other hand, a mCCI≥5 predicts the same outcome with a sensitivity/specificity of 26%/88%. The diagnostic odds ratio for CFS≥5 was 11.6, compared to only 2.7 for mCCI≥5. Lastly, we constructed a model in which both scores interacted (as categorical variables), which after multivariable adjustment showed that mCCI/CFS <5/≥5 and ≥5/≥5 were independent predictors of mortality (HR=7.06; P=0.017; HR=10.708; P=0.002; respectively). Interestingly, no events were observed in the mCCI≥5/CFS<5 group.
Conclusions: In this cohort of urgent-start incident HD patients, frailty defined by CFS was a stronger predictor of mortality than comorbidity defined by CCI.
Keywords: frailty, comorbidity, elderly, dialysis, chronic kidney disease
Despite the considerable proportion of health care resources being used, morbidity and mortality remain high in end-stage kidney disease (ESKD) patients especially during the first months on dialysis therapy.1–3 Comorbidity and frailty, both frequent in elderly patients with chronic kidney disease (CKD), are associated with a higher risk of death even irrespective of age and dialysis need.4–7
Frailty is a measure of increased vulnerability to stressful situations caused by reduced functional biological reserves.8 This condition raises the risk of progression of disease, disability, hospitalization, and death.9 There are many tools to assess the degree of frailty, clinical frailty scale (CFS) is a simple yet global frailty assessment, proved as an independent predictor of mortality in dialysis patients.10–12 The Charlson Comorbidity Index (CCI), a scale used to assess and quantify comorbidity burden, has also proved to have prognostic value in a variety of conditions as CKD.5,13,14 Frailty and comorbidity are often presumed to be close concepts however they don’t always coexist, even sometimes traducing opposite information about the same patient. We aimed to access the predictive value of frailty and comorbidity to predict early death in urgent start hemodialysis patients.
We conducted a retrospective cohort study of patients aged 65 years and over, referred to the Nephrology Department of Centro Hospitalar Universitário do Porto (CHUPorto), who underwent urgent start hemodialysis as their first renal replacement therapy (RRT), between January 2014 and December 2020. CHUPorto is a tertiary care hospital, which serves a diverse population of 500,000 inhabitants in the Northern region of Portugal. Urgent start hemodialysis was defined as any first treatment started for an emergency condition or not appropriate to delay for >24 h, even if a permanent dialysis access was in place.
Data were collected from medical records and included (at dialysis initiation): sex, age, associated comorbid conditions, such as diabetes, dyslipidemia, hypertension, smoking status, history of malignancy, and cardiovascular disease. Variables related to renal care included vascular access (graft/fistula versus catheter) and the timing of nephrologist care referral prior to dialysis. Glomerular filtration rate (GFR) was estimated using the CKD Epidemiology 2021 creatinine equation15; all serum creatinine measurements were performed in the same laboratory calibrated using a calibrator for automated systems (Roche Diagnostics).
A modified version of the Charlson comorbidity index (mCCI), by excluding subject’s age and presence of kidney disease, was used to evaluate comorbidity. Frailty was defined by the Clinical Frailty Scale that evaluates specific domains including comorbidity, function, and cognition to generate a frailty score ranging from 1 (very fit) to 9 (terminally ill).
The study outcome was all-cause mortality within first 6 months following dialysis therapy initiation. Vital status was checked until August 30, 2021.
Continuous data were described using mean (standard deviation) or median (interquartile range) and categorical data were expressed as numbers (frequencies). Categorical were compared using Pearson χ 2 test or Fisher's exact test, as appropriate. Continuous variables were compared with Student t-test or Mann–Whitney U test), as appropriate. Receiver operator characteristic (ROC) curves and area under the curve (AUC) were calculated for CFS and mCCI scores, as well as sensitivity, specificity, positive and negative diagnostic odds ratios. Graft survival curves were visualized using Kaplan–Meier method, with comparison groups being done by log-rank test. Association between frailty and comorbidity index (and their interaction) and early death was performed by multivariable Cox regression model.
A two-sided P-value of <0.05 was considered statistically significant. Statistical calculations were performed using Stata/MP, version 15.1 (Stata Corp, College Station, TX).
Study population
During the study period, 166 patients with 65 years old or more met the criteria of urgent-start HD. Patient demographics are shown in Table 1. The median age was 75.5 years, 58% were men. Common comorbidities included hypertension (97%), dyslipidemia (93%), congestive heart failure (72%), diabetes (54%) and peripheral vascular disease (51%).
|
Total |
Clinical frailty scale |
p value |
Alive at 6-months |
p value |
||
|
N=166 |
<5 N=62 (37%) |
≥5 N=104 (63%) |
Yes N=135 (81%) |
No N=31 (19%) |
||
Age (years), mean±sd |
75.5±6.3 |
73.9±5.4 |
76.4±6.6 |
0.016 |
75.3±6.0 |
76.1±7.2 |
0.54 |
Gender, female, n (%) |
69 (42) |
21 (34) |
48 (46) |
0.12 |
55 (41) |
14 (45) |
0.652 |
Body mass index (kg/m2), mean±sd |
24.0±6.0 |
24.3±5.9 |
23.9±6.1 |
0.689 |
24.3±5.4 |
22.7±7.9 |
0.174 |
Diabetes mellitus, n (%) |
90 (54) |
23 (37) |
67 (64) |
0.001 |
73 (54) |
17 (55) |
0.939 |
Hypertension, n (%) |
161 (97) |
60 (97) |
101 (97) |
1 |
132 (98) |
29 (94) |
0.234 |
Dyslipidemia, n (%) |
155 (93) |
56 (90) |
99 (95) |
0.222 |
127 (94) |
28 (90) |
0.432 |
Nephrology referral <4months, n (%) |
26 (16) |
9 (15) |
17 (16) |
0.754 |
16 (12) |
10 (32) |
0.005 |
Central venous catheter, n (%) |
107 (64) |
35 (56) |
72 (69) |
0.096 |
82 (61) |
25 (81) |
0.037 |
eGFR at dialysis start (ml/min), mean±sd |
8.3±4.2 |
7.1±3.1 |
9.0±4.7 |
0.005 |
8.5±4.4 |
7.2±3.1 |
0.141 |
Albumin serum (g/dL), mean±sd |
3.3±0.58 |
3.43±0.58 |
3.29±0.58 |
0.14 |
3.39±0.59 |
3.16±0.53 |
0.049 |
Coronary heart disease, n (%) |
62 (37) |
16 (26) |
46 (44) |
0.018 |
46 (34) |
16 (52) |
0.069 |
Heart failure, n (%) |
119 (72) |
35 (56) |
84 (81) |
0.001 |
96 (71) |
23 (74) |
0.731 |
Cerebrovascular disease, n (%) |
49 (30) |
10 (16) |
39 (38) |
0.003 |
42 (31) |
7 (23) |
0.348 |
Peripheral artery disease, n (%) |
84 (51) |
22 (35) |
62 (60) |
0.003 |
67 (50) |
17 (55) |
0.601 |
Neoplasm diagnosis <5years, n (%) |
36 (22) |
14 (23) |
22 (21) |
0.829 |
30 (22) |
6 (19) |
0.727 |
CFS, median (IQR) |
5 (4-6) |
3 (3-4) |
6 (5-7) |
- |
5 (4-6) |
6 (5-7) |
0.001 |
CFS≥5, n (%) |
- |
- |
- |
- |
75 (56) |
29 (94) |
<0.001 |
mCCI, median (IQR) |
4 (3-6) |
3 (2-4) |
5 (4-7) |
<0.001 |
4 (3-6) |
5 (4-7) |
0.037 |
mCCI≥5, n (%) |
80 (48) |
15 (24) |
65 (63) |
<0.001 |
59 (44) |
21 (68) |
0.016 |
Table 1 Patient demographics stratified by clinical frailty scale and life status at 6 months
Comorbidity and frailty
Figure 1 shows the distribution of CFS and mCCI scores. The median CFS was 5 (inter quartile range 4-6) The median mCCI was 4 (inter quartile range 3-6).

Figure 1 Distribution of the mCCI and CFS in the cohort. mCCI, modified Charlson comorbidity index; CFS, clinical frailty scale.
Considering 6-month mortality prediction, the performance of CFS was superior to the mCCI (p=0.031; Figure 2). In fact, the area under the curve (AUC) was considerably higher in CFS (0.739) versus the mCCI (0.620). For both scores, the analysis of receiver operating characteristics curve, established the optimal cut-off to be ≥ 5points. A CFS≥5 had a sensitivity/specificity of 94%/44% in prediction the primary outcome, respectively. On the other hand, a mCCI≥5 predicted the same outcome with a sensitivity/specificity of 26%/88%. The diagnostic odds ratio for CFS≥5 was 11.6, compared to only 2.7 for mCCI≥5. The group of frail patients (CSF ≥5) were older (p=0.016), started dialysis with a higher eGFR (p=0.005) and had more comorbidities (p<0.001) (Table 1).
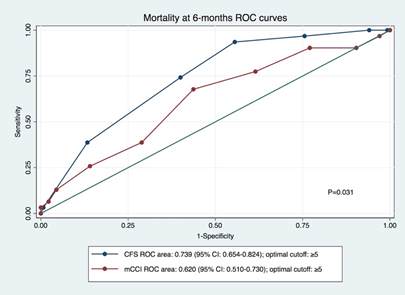
Figure 2 Area under the Receiver operating characteristic curves by mCCI and CFS. mCCI, modified Charlson comorbidity index; CFS, clinical frailty scale.
Survival analysis
Nineteen percent of the patients died during the follow up period. Table 1 shows the clinical and demographic data, accordingly vital status. In this cohort, age was not significantly different (p=0.540), while later referral to nephrology (p=0.005), lower serum albumin (p=0.049) and catheter as vascular access (p=0.037) were more common in those who died. Overall survival rate at 3/6-months was 89/81%, respectively.
Patients’ survival curves by CFS (≥5 vs <5 at 3-/6-months, respectively: 83%/72% vs 98%97%; P<0.001) and mCCI (≥5 vs <5 at 3-/6-months, respectively: 85%/74% vs 92%/88%; P=0.020) groups are depicted in Figure 3 A & B, respectively. Survival curves stratified by both scores are shown in Figure 4, both groups with CFS≥5 had worse survival, independently from the mCCI score.
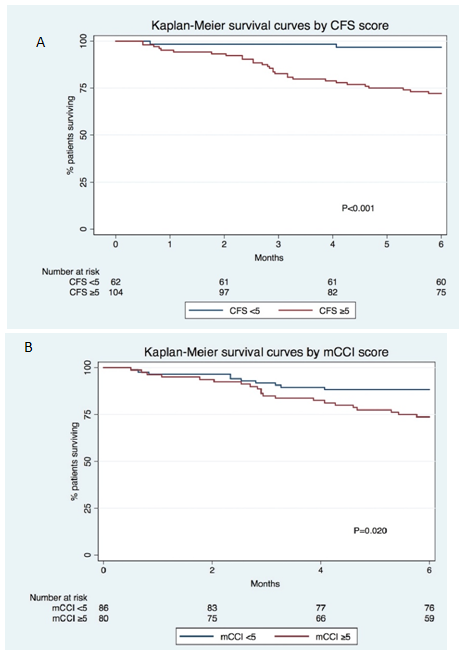
Figure 3 Kaplan Meier survival curves by CFS (A) and mCCI (B). mCCI, modified Charlson comorbidity index; CFS, clinical frailty scale.
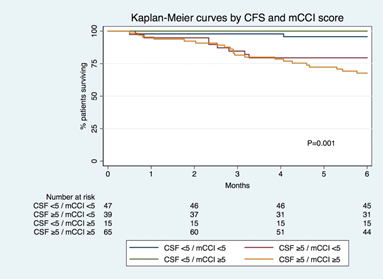
Figure 4 Kaplan-Meier survival curves by groups defined by the interaction of mCCI and CFS. mCCI , modified Charlson comorbidity index; CFS, clinical frailty scale.
In a multivariable adjusted cox model (Table 2), CFS was an independent predictor of 6-month mortality both as a categorical (CSF≥5) (HR=3.64; P=0.004) and continuous variable (HR=1.95; P<0.01); while mCCI was not. When groups defined by the interaction of both scores were analyzed (CFS/mCCI <5/<5 as reference), in group 1 (mCCI≥5/CFS<5) no events were observed, while the remaining groups (mCCI>5/CFS≥5, mCCI≥5/CFS≥5) were independent predictors of mortality.15
|
Alive at 6-months |
p value |
|
|
Yes N=135 (81%) |
No N=31 (19%) |
|
Age (years), mean±sd |
75.3±6.0 |
76.1±7.2 |
0.54 |
Gender, female, n (%) |
55 (41) |
14 (45) |
0.652 |
Body mass index (kg/m2), mean±sd |
24.3±5.4 |
22.7±7.9 |
0.174 |
Diabetes mellitus, n (%) |
73 (54) |
17 (55) |
0.939 |
Hypertension, n (%) |
132 (98) |
29 (94) |
0.234 |
Dyslipidemia, n (%) |
127 (94) |
28 (90) |
0.432 |
Nephrology referral <4months, n (%) |
16 (12) |
10 (32) |
0.005 |
Central venous catheter, n (%) |
82 (61) |
25 (81) |
0.037 |
eGFR at dialysis start (ml/min), mean±sd |
8.5±4.4 |
7.2±3.1 |
0.141 |
Albumin serum (g/dL), mean±sd |
3.39±0.59 |
3.16±0.53 |
0 .049 |
Coronary heart disease, n (%) |
46 (34) |
16 (52) |
0.069 |
Heart failure, n (%) |
96 (71) |
23 (74) |
0.731 |
Cerebrovascular disease, n (%) |
42 (31) |
7 (23) |
0.348 |
Peripheral artery disease, n (%) |
67 (50) |
17 (55) |
0.601 |
Neoplasm diagnosis <5years, n (%) |
30 (22) |
6 (19) |
0.727 |
CFS, median (IQR) |
5 (4-6) |
6 (5-7) |
0.001 |
CFS≥5, n (%) |
75 (56) |
29 (94) |
<0.001 |
mCCI, median (IQR) |
4 (3-6) |
5 (4-7) |
0.037 |
mCCI≥5, n (%) |
59 (44) |
21 (68) |
0.016 |
Table 2 Patient demographics stratified by life clinical frailty Scale
CFS, clinical frailty scale; eGFR, estimated glomerular filtration rate; IQR, inter quartile range; mCCI - modified version of the Charlson comorbidity index; SD, standard deviation
In this study, we found that frailty was a better predictor of early mortality in urgent-start hemodialysis patients.
Whereas the best definition and classification of frailty and comorbidity remain a matter of discussion, it is well recognized that they entail a higher risk of unfavorable outcomes such as hospitalization and death.9,16 Despite the higher risk of death by virtue of having CKD, frailty and comorbidity were associated with increased mortality. In our study, this association persisted after adjustment for age, sex, body mass index, serum albumin, vascular access and estimated glomerular filtration rate at dialysis start (Tables 3 & 4).
Total |
Clinical frailty scale |
p value |
||
|
N=166 |
<5 N=62 (37%) |
≥5 N=104 (63%) |
|
Age (years), mean±sd |
75.5±6.3 |
73.9±5.4 |
76.4±6.6 |
0.016 |
Gender, female, n (%) |
69 (42) |
21 (34) |
48 (46) |
0.12 |
Body mass index (kg/m2), mean±sd |
24.0±6.0 |
24.3±5.9 |
23.9±6.1 |
0.689 |
Diabetes mellitus, n (%) |
90 (54) |
23 (37) |
67 (64) |
0.001 |
Hypertension, n (%) |
161 (97) |
60 (97) |
101 (97) |
1 |
Dyslipidemia, n (%) |
155 (93) |
56 (90) |
99 (95) |
0.222 |
Nephrology referral <4months, n (%) |
26 (16) |
9 (15) |
17 (16) |
0.754 |
Central venous catheter, n (%) |
107 (64) |
35 (56) |
72 (69) |
0.096 |
eGFR at dialysis start (ml/min), mean±sd |
8.3±4.2 |
7.1±3.1 |
9.0±4.7 |
0.005 |
Albumin serum (g/dL), mean±sd |
3.3±0.58 |
3.43±0.58 |
3.29±0.58 |
0.14 |
Coronary heart disease, n (%) |
62 (37) |
16 (26) |
46 (44) |
0.018 |
Heart failure, n (%) |
119 (72) |
35 (56) |
84 (81) |
0.001 |
Cerebrovascular disease, n (%) |
49 (30) |
10 (16) |
39 (38) |
0.003 |
Peripheral artery disease, n (%) |
84 (51) |
22 (35) |
62 (60) |
0.003 |
Neoplasm diagnosis <5years, n (%) |
36 (22) |
14 (23) |
22 (21) |
0.829 |
CFS, median (IQR) |
5 (4-6) |
3 (3-4) |
6 (5-7) |
- |
mCCI, median (IQR) |
4 (3-6) |
3 (2-4) |
5 (4-7) |
<0.001 |
mCCI≥5, n (%) |
80 (48) |
15 (24) |
65 (63) |
<0.001 |
Table 3 Patient demographics stratified by life status at 6 months
CFS, clinical frailty scale; eGFR, estimated glomerular filtration rate; IQR, inter quartile range, mCCI - modified version of the Charlson comorbidity index; SD, standard deviation
|
Hazard ratio |
95% CI |
P |
Model 1 (categorical variables) |
|||
mCCI ≥5 |
1.371 |
0.610-3.081 |
0.445 |
CFS ≥5 |
10.374 |
2.294-46.921 |
0.002 |
Model 2 (continuous variables) |
|||
mCCI |
0.95 |
0.754-1.198 |
0.665 |
CFS |
1.944 |
1.349-2.802 |
<0.001 |
Model 3 (mCCI/CFS) |
|||
<5/<5 |
Reference |
- |
- |
≥5/<5 (group 1) |
No events |
- |
- |
<5/≥5 (group 2) |
7.068 |
1.414-35.320 |
0.017 |
≥5/≥5 (group 3) |
10.708 |
2.465-46.514 |
0.002 |
Table 4 Multivariable adjusted cox model of CFS and mCCI as mortality predictors (adjusted for age, sex, body mass index, vascular access, late referral to nephrology, serum albumin and estimated glomerular filtration rate at dialysis start)
CI, confidence interval; mCCI, modified version of the Charlson comorbidity index; CFS, clinical frailty scale
Frailty and comorbidity were frequent in our population, in congruence with similar cohorts.17–23 Almost two thirds of the patients were frail, probably because CKD is a risk factor to frailty but also because these patients had a high burden of comorbidities. Some symptoms associated with comorbidities, such as weakness and slowness, can prompt physicians to misdiagnose a frailty phenotype, on the other hand it is also likely that these comorbidities can weaken patient’s biological reserves.24 We confirmed that mCCI and CFS are independent predictors of mortality, CFS as a categorical and continuous variable but mCCI only as a categorical variable. The increased risk of death with continuous increase in the CFS score emphasizes the potential prognostic significance of frailty. Our findings are comparable with those of Rockwood et al and Alfaadhel et al, both observed a similar increased risk of death.11,25 This risk increase was not achieved by mCCI.
In this cohort of incident hemodialysis patients aged 65 years and over, almost a fifth died within the first 6 months of follow-up. These results are slightly superior to those found in previous studies.26–29 We can speculate about the reasons for this difference, namely our patients were older and demanded urgent start hemodialysis which are two known conditions for worse outcomes. Nonetheless, age at dialysis start, did not modified the association between frailty, comorbidity, and death. This finding was reported in some other studies and shows that additional factors influence patient’s outcomes regardless of age.7,30
We intended to access the ability of mCCI and CFS to predict mortality but also to evaluate the interaction between these scores. Both scores had a DOR higher than 1, but CFS≥5 DOR was 4 times higher than mCCI≥5. Also, Kaplan-Meier analysis by CFS and mCCI shows earlier and more expressive separation of the curves in the CFS compared to mCCI. These data support that CFS is a better score to predict early mortality versus the mCCI. Frailty ability to predict mortality is evident even in the cases where mCCI traduces low comorbidity burden as showed by the model in which both scores interacted as categorical variables. Is also important to point out that the group with CFS and mCCI <5 had almost 100% survival rate at 3 and 6 months proving the ability of these scores to predict not only death but also good outcomes.
The exact reason why frailty predicts mortality better then comorbidity is unknown. Frailty evaluates the level of physiologic reserves of one individual that may be decreased by their comorbidities, sometimes despite the high burden of diseases the functional status is preserved. Our study pointed out that the repercussion of the diseases is a better determinant of early death than the diseases alone.
This work has also some limitations. First, it is a single-center study with a limited number of patients. Despite the effort to adjust the results, the observational nature of the study might allow the existence of unmeasured and unknow confounders that influence the relation between frailty, comorbidity, and death. CFS is an impression-based frailty score, its subjective nature might promote the existence of some bias. It can also be argued that instead of comparing frailty and comorbidity we compared two different scores that might not properly evaluate frailty and comorbidity. Nonetheless, we choose two scores that are already extensively validated in hemodialysis patients.6,10,11,31–33 Finally, as one time acquisition of the scores also enables the misclassification of the patients, longitudinal evaluation of frailty and comorbidity could improve the accuracy of data collection and limit the misclassification.
In conclusion, we identified that frailty and comorbidity defined by CFS and mCCI are associated with an increased early mortality risk on incident dialysis patients. We also proved frailty to be a more accurate predictor of early mortality versus comorbidity especially in the first three months. Further studies should be held to validate these conclusions which supports that decisions related with RRT should be based on frailty evaluation instead of chronological age and or/comorbidity alone.
None.
The authors report no conflicts of interest in this work.
None.

©2022 Fernandes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.