MOJ
eISSN: 2573-2919


Research Article Volume 7 Issue 2
1Department of Environmental Sciences, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Pakistan
2Department of Pharmaceutical Chemistry, Omar Almukhtar, University Libya
3Faculty of pharmaceutical sciences Riphah International University Islamabad, Lahore campus, Pakistan
4Department of Botany the Islamia University Bahawalpur, Pakistan
5Department_ School of Public Health - Dow University of Health Sciences Karachi Pakistan
6Department of Environmental Science, COMSATS University, Islamabad, Abbottabad Campus Pakistan
Correspondence: Tehreem Lutaf Ullah, Department of Environmental Sciences, Pir Mehr Ali Shah Arid Agriculture University Rawalpindi, Punjab, Pakistan
Received: March 15, 2022 | Published: April 8, 2022
Citation: Ullah TL, Mahmood T, Elhddad S, et al. Identification of plants releasing isoprene causing smog. MOJ Eco Environ Sci. 2022;7(2):40-46. DOI: 10.15406/mojes.2022.07.00245
The accessibility of water is most important component for plant productivity and growth. Water stress is a major threat for the agriculture system of Pakistan. The change in climate causes the change in the patterns of rainfall which may cause the extreme events. The main cause of climate change is the concentration of carbon dioxide in the air and global warming. A change in environment overall takes place by a number of causes but the most important cause of all these changes is isoprene.1 Water scarcity causes high isoprene emission from plants. The dominant volatile organic compound which is released from vegetation is Isoprene (2-methyl-1,3,-butadiene). Isoprene produced by few herbaceous plant species and many woody trees. The emission of these volatiles like isoprene has an indirect effect on climate change and cause smog formation. The examination found that isoprene responds with human made nitrogen oxide to make particulate issue. In light of the presence of nitrogen oxides, it is engaged with creating the negative impacts on climate and human wellbeing. The research investigates current knowledge about the presence of isoprene emissions within the plant kingdom under drought stress conditions. Eucalyptus, Kachnar, Mulberry and Conocarpus are few species which are able to produce isoprene. The pot experiment was designed to identify isoprene emission within these plants. The plants were exposed to decrease Fractional Transpirable Soil Water FTSW. Various VOCs emission was increase under stress conditions. The isoprene emission rate is high under mild drought stress but decline under severe drought stress. Other parameters like stomatal conductance, photosynthesis, internal CO2 were also observed.
Keywords: index terms-, isoprene, smog, GC-FID
Given the potential impacts, climate change is always a concern for scientists. Increased environmental temperature and carbon dioxide are shown to be factors in the gradual climate change.1 The Increase in carbon dioxide concentration in atmosphere has been detected in the past due to human activities like urbanization. It is observed that carbon dioxide amount will increase up to 750 ppm at the end of this era. The earth’s temperature has been increased to 0.4 - 0.660c in the present due to increase in CO2.1 Environmental changes have resulted in variations in rainfall patterns, magnitude and rate, resulting in irregular, low and unpredictable rainfall patterns that result in availability of water at native and geographical levels. Further, the current global change example promotes regional drought by predicting rising temperatures and prolonged drought with less rainfall.2,3
Most significant components for the growth of plant and for their biochemical functions are water and CO2.4 Carbon uptake is affected by the enlarged amount of CO2 during the cellular gas exchange process, whereas a low availability of water prevents physiological functions such as the exchange of leaf gases, light synthesis and exchange of leaf gases, which eventually lead to prevent the growth and productivity of plants. The shortage of water is going to be high in the future. It will cause climate change and drought conditions in various parts of the world, which has become an important factor in limiting plant performance, leading to improved plant efficiency and production of biomass decreases.5
During conditions of drought stress, the plants absorb smaller amount of water than transpiration and if this situation continues they target plant by damaging their growth, number of leaves, leaf area, fading, cell membranes and eventually lead to plant death. The main effect of limited supply of water to the plant trunk is partial or complete closing of the stomata, which generally reduces the conversation of water and carbon dioxide with the exterior environment. Less stomata conductivity decreases the level of internal carbon dioxide for photosynthesis. Low water availability also reduces photosynthesis process by decreasing in the area of leaf and photo unit rate unit per area.
Limited or high drought conditions affect the gaseous exchange and dynamics of carbon in plants in the ecosystem.6 To cope with stress situations, a large amount of volatile compounds are discharge by plants into the environment.7 During common circumstances, factories discharge about 3% saturated carbon in the manufacture of Biological Volatile Organic Compounds. Though, specifically in the case of drought stress, the plants are given carbon above 11% in the form of VOCs.8
Biological volatile organic compounds are a huge group of volatile compounds, with almost 1, 1,700 substances obtained from the plant identified.9 These compounds include a large variety of organic species, which includes isoprene, hemiterpenes, terpenes, monoterpenes, isoprenoids, and compounds of oxygen.10 There are many plants which released great amounts of isoprene. Eucalyptus, Wild Mulberry, Kachnar and Conocarpus are among the few species of plants that can release isoprene as well as monoterpenes. Monoterpenes are present in huge amount are usually kept in the tanks, while isoprene is present in free form and not in stored form, that’s why it is openly released into the environment. The worldwide natural source rate is estimated per year is 1.1 to 1.6Pg Carbon11 and this amount is much higher than human productions minutes.12
Biocompatible organic compounds (BVOCs) originated in marine and terrestrial ecosystems. About 80% of volatile compounds are spread by trees and plants, in which the most important species are monoterpenes and isoprene. Deep farming programs started in 1979 to improve plants in India in 2005. In these programs different plant species were grown in different areas including rural and urban. The extensive cultivation of plant types offers much welfare, such as improving the quality of air, lower air temperatures, increasing soil fertility and isolating carbon dioxide. Still, several plant species have been emitting extremely volatile isoprene amount.13
Isoprene produces and disperses many types of wood and is one of the main reactors of tropospheric chemistry in continental regions.14 Isoprene excretion cannot be detected in the dark, but starts faster when illuminated and causes fully stimulated genomic tissue interaction within 25 minutes.15 All environmental dynamics (light, temperature, water stress, etc.) and the organism genetic structure have been studied. In dense vegetation areas, these volatile organic compounds direct the oxidizing atmosphere capacity which produces smog16 and secondary organic aerosols.
There are many plants which released great amounts of isoprene. Eucalyptus, Wild Mulberry, Kachnar and Conocarpus are among the few species of plants that can release isoprene as well as monoterpenes. Monoterpenes are present in huge amount are usually kept in the tanks, while isoprene is present in free form and not in stored form, that’s why it is openly released into the environment. Many people living in the semi-hilly areas of Pakistan, including Islamabad, are familiar with "Kachnar". It is from Pakistan and grows in large quantities in tropical mountainous areas. Kachnar (Bauhinia Variegate) can withstand very stressful situations. Therefore, minimal isoprene is emitted.
Eucalyptus also known as Blue Gum was brought to Pakistan from Australia a hundred years ago. This is an evergreen aromatic tree. Nowadays, it is widely cultivated in many areas of Punjab including Mansehra etc. The negative effects have recently been recognized in Pakistan. Eucalyptus spp. isoprene is one of the top plants that discharge isoprene. These plants usually grow along the coast and deal with the usual salt stress. Photosynthesis is inhibited under water pressure and dehydration, and transport traffic to carbon dioxide is gradually reduced.17 When performance of photosynthesis restored, then a wave of isoprene emission occurs.
Wild Mulberry (Morus Alba), a faster growing plant, is known as wild mulberry. Mulberry tree is grown in some parts of Pakistan. However, it does have some negative effects on the ecosystem. Like Eucalyptus in stressful situations, it emits isoprene. Severe drought effect carbon dynamics and plant gas exchange.6,18 Under dehydration conditions, mulberry release a large amount of VOC in the atmosphere.7 However, the plant gives above 11% of the same carbon in the usage of volatile compounds, especially in case of water stress.8
The objective of this research was to identify and compare the isoprene emission rate in the Eucalyptus, Wild Mulberry, kachnar and Conocarpus under water stress conditions.
This experimental study was directed to evaluate the emission of isoprene from different plants species (Eucalyptus, Wild Mulberry, Conocarpus, Kachnar). Six replications of each plant were taken. The isoprene emission was monitored under drought stress.
Plant selection
Plant species Eucalyptus, Conocarpous, Mulberry and Kachnar were selected for this experimental study because of their local availability and wide abundance. The selected plants were obtained from local nurseries. Six plants from each specie have been selected for this study.
The plants of uniform height and several growth stages of leaves (12-15) were selected and then shifted them into ceramic pots (18.0cm diameter and 15.3cm deep). Pots were contained the fertile garden soil mixes with organic manure in the ecological garden.
Physiological analysis of plants
Effect of drought stress and gas exchange measurements were done to evaluate the physiological response of the plants Eucalyptus, Kachnar, Wild Mulberry and Conocarpus. Measurements of these plants were carried out on the daily basis.
Isoprene Sample Collection and analysis
Sample of Isoprene were taken from fresh fully expanded and mature leaves of all plants (Eucalyptus, Conocarpus, Wild Mulberry, Kachnar). Isoprene collection was performed three times in pot experiments (1. Full irrigated condition, 2. Under Mild stress condition when there was decrease in stomatal conductance 3. Under Severe stress when stomatal conductance reached zero). Two different experiments were performed.
Experiment (1)
The first experiment was carried out at the beginning of autumn. In this experiment all the plants (Eucalyptus, Conocarpus, Wild Mulberry, Kachnar) were well watered and a maximum amount of water was endorsed for drainage overnight. Initial and final pot weight was measured on a digital balance. Twelve plants were under water-stress and withholding water, while other 12 plants were fully irrigated up to the capacity of pot capacity which represented as control plants. Each plant was then enclosed in a plastic bag and slightly tightened with gardening wire (Figure 3.5). The sample was taken by using air tight syringe. These samples were collected in vials and mixed with methanol. Samples were collected for 1 h and 30 min. And they were analyzed by using GC-FID.
Experiment (2)
The experiment was carried out at the start of winter. For this experiment, the isoprene concentration was measured and expressed on the basis of leaf area. A leaf meter was used to measure a leaf area. The reagents are given below for this experiment:
After measuring the area, the 4-5 leaves per plant were separately dried in oven at 500oC and then with the addition of nitrogen they were ground to powder and consequently transferred into simple vials (15mL). Then we put the vials in water bath and incubate for 10 min at 370C. Add 1.1 g solid calcium chloride (CaCl2) and 0.5 ml EDTA (100mM, pH 7.5). Then close the vial and check vigorously. Now shake for 5 min in shaker incubator to facilitate CaCl2. Once this is done, transfer 900µl of the homogenize mixture to the bottom of 10 headspace vials. Now the sample is stable for up to 12 hours.
Isoprene analysis
The vials were analyzed by using GC-FID. The GC has been prepared by a split less injector and a HP-5MS capillary column (30m in length, 200m i.d. and 0.4m film thickness) and coupled with a mass selective detector. The oven temperature of column has been kept at 30ºC for the first 1 min, after that the temperature was increased to 60ºC to 150ºC in the next min and finally it was sustained at 250ºC for 7 min. A carrier gas He (Helium) was used for this purpose. Standards of gas at a concentration of 1 mg α- Pinene and Lamonene at a concentration of 100ppb were being used for the calibration of GC system. Then the isoprene concentration was estimated by direct comparison with the gaseous standards peak area. The identification of compound was seen by using the GC Chem Station software (Agilent). By analyzing the main fragments on spectra and parent ions, the retention time of GC peak was substantiated.
Statistical analysis
Statistical analysis (Univariate Analysis of Variance) was used for the analysis of all data set treatments. By using least significant differences (LSD) the mean values were calculated to determine the significant differences among all treatments.
The water stress is the main reason of decrease in leaf conductance and in the plant biochemical procedures which shows that isoprene emission is actually an inhibitory factor for plant physiological responses. Different physiological responses a stomatal conductance, rate of photosynthesis and transpiration, an internal carbon dioxide and chlorophyll content were observed in selected plants during this experiment.
Physiological analysis of plants
The physiological responses of plants were analyzed under drought stress. The rate of photosynthesis, transpiration rate, stomatal conductance and internal carbon dioxide was similar at the middle of drought stress. A sharp decline was observed at severe stress condition.
Isoprene emission
Isoprene emission was identified under drought stress condition (Figures 1–4). Isoprene emission was identified at early, middle and final stage of drought stress period (FTSW 100%, FTSW 50% and FTSW<10%). With the increase in drought stress the isoprene emission is also increased. In this experiment the full irrigated plants released a small amount of isoprene as compared to stressed plants. Different plants showed different emission rates at different stress periods.
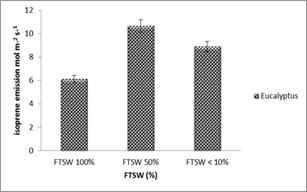
Figure 1 Isoprene emission from leaves of Eucalyptus spp. under drought stress (n=3) ± SD. Three levels 100%, 50%, < 10 of Fractional of Transpirable Soil Water (FTSW) were made.
The plants released small number of isoprene in the beginning of drought stress period. In the middle of the drought stress a large number of isoprene emission rates were measured. But a slight decline at the end of this experiment when stress rate was on peak (FTSW<10) was observed. However, higher emission rate was observed in Eucalyptus spp. under drought stress period.
At FTSW 50% large number of isoprene emission was observed in all plants. At FTSW<10% the isoprene was still emitted in many stressed leaves of plants. A minor significant difference (P<0.05) was detected in all treatments. Other major and minor compounds were also identified during this experiment i.e. Limonen and α-pinene increased with decreasing FTSW.
Water is an important factor which takes part in biochemical procedures and therefore it is necessary for growth of plant. The water stress effects the plant development and growth and brings normal plant into stress. Under drought conditions the physiological changes have been identified in plants. We found that plants produce secondary volatile compounds under drought stress.
Under drought stress conditions, the plant physiological responses were identified. Plants were observed for their responses to different stress conditions. We found that water scarcity causes a decline in leaf stomatal conductance, photosynthesis, water use efficiency and transpiration rate, which causes to low carbon assimilation and plant growth.
Stomatal conductance is indicator of water scarcity and that’s why as compared to other parameters it is quickly impacted by the water shortage.19 In our study the stomatal conductance was decreased due to increase in drought stress. The stomatal conductance rate of Eucalyptus and Kachnar was high as compared to other two plants.
Photosynthesis is important for plant yield. Photosynthesis is inhibited under drought stress.20 Its rate can be affected by the biochemical limitations. Under water stress conditions the photosynthesis rate is inhibited by closing of stomata. However, due to closing of stomata the reduction takes place in internal CO2 which decreases the carbon assimilation rate.21 In this study the photosynthesis reduction takes place in all plants due to drought stress. But the photosynthesis rate was high and stable in all controlled plants. So, the past study showed that the closing of stomata does not limit the photosynthesis rate under stress condition.
The study also indicated that the rate of transpiration reduced due to decrease in stomatal conductance under drought stress. The Conocarpus and Mulberry showed a high transpiration rate over other plants. The transpiration is also affected by the regulation in stomatal conductance under water stress.22 The closing of stomata decrease the transpiration rate but increase the water use efficiency.
The reduction in chlorophyll content in plants has been observed under drought stress. The chlorophyll content of Kachnar and Conocarpus decreased slowly relative to other plants for the first 12 days.
Under drought situations, large amount of assimilated carbon is released in the form of BVOCs by plants.9 A major emitting group which is the sum of volatile emission is isoprenoid (a major emittors in plants). Isoprene acts as a defensive compound of plant against high stress situation.7 Earlier studies of biogenic volatile compounds revealed that the effect of drought stress on isoprene emission is directly proportional to the stress intensity.23 The minor rates of drought stress do not stimulate the monoterpene and isoprene emissions.
In our experimental studies, the isoprene emission rate was too low to identify in our first pot experiment during drought condition, whereas in our second pot experiment isoprene emission rate become high at middle of an experiment and uncoupled from photosynthesis. Isoprene emission rate continued to increase even when the photosynthesis rate declined. Probably in the case of un-coupling a huge amount of carbon from alternative sources subsidized in maintaining the high emission rate of isoprene as a rate of photosynthesis declined. Relation among high rate of isoprene emission and a declined in photosynthesis rate showed that the isoprene emission is less delicate to water shortage as related to photosynthesis and stomatal conductance, is unaffected during water scarcity and therefore, it becomes disengaged from rate of photosynthesis during drought stress. In the experiment, the production rate of isoprene constantly increased during drought stressed situation as a very low quantity of energy require for a leaf ontogeny and production of isoprene. Mature leaves released large amount of isoprene. This increase in the emission rate of isoprene during drought stress conditions can be recognized as the increased in transport of electron - net assimilation rate which increased availability by decreasing the methylerythritol phosphate (MEP) corridor in other sinks of non- photosynthetic carbon reduction.
Emission rate of Isoprene declined and lastly stopped during persist high drought situations.24 The emission rate of isoprene was reserved and decreased under severe water stress condition, which possibly cause a photochemistry failure in plants. The failure would be happened only during prolonged drought stress conditions over the endpoint of FTSW, at this point stomata become totally closed for the prevention of plant dehydration.25,26–103
In the present study we concluded that in the first pot experiment (branch enclosure method) for isoprene measurement, only a small concentration of isoprene was observed that was non-significant in all treatments. In second experiment the isoprene emission was high in drought stressed plants as related to control plants, but the other VOCs compounds did not affect significantly. High isoprene emission was observed at middle of the stress and later showed decrease at the end (FTSW<10%) of experiment. The stress and later showed decrease at the end (FTSW<10%) of experiment.
None.
None.
Authors declare that there is no conflict of interest.

©2022 Ullah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.