MOJ
eISSN: 2374-6912


Research Article Volume 2 Issue 2
1Department of regenerative medicine, Clinica Medica Quirurgica Quantum, Spain
2Department of Research and Development, Stemtech International, USA
3Head Team Doctor, Madrid, Spain
Correspondence: Garber Miguel Guillermo, Department regenerative medicine, Clinica Medica Quirurgica Quantum, Clara del Rey 31-33, Madrid, 28002, Spain, Tel +34628766753
Received: March 09, 2015 | Published: April 29, 2015
Citation: Garber M, Mazzoni P, Nazir C, et al. Use of stem cell mobilizer se2® and the circulation enhancer stemflo® as part of conventional treatment on ankle injuries to expedite recovery in professional soccer players. MOJ Cell Sci Rep. 2015;2(2):38-41. DOI: 10.15406/mojcsr.2015.02.00023
Sport injuries are ubiquitous in both professional and amateur sports. In professional sport, injuries have significant financial impacts and one of the main goals of sports medicine is to reduce the healing time and improve recovery in order to shorten off match time. The most common injuries are muscle and ligaments sprains. Peripheral blood stem cells have been shown to have the ability of migrating into muscles and other soft tissues, and to participate to the process of tissue repair. In fact, increasing the number of circulating stem cells has been shown to improve and accelerate the healing of various tissues, including muscles and ligaments. In this study, 12 professional soccer players with soft tissue ankle injuries were pair-matched and distributed into two groups, control and experimental. In addition to traditional treatment received by both groups, the experimental group consumed the natural stem cell mobilizer SE2® and the circulation enhancer StemFlo®. Players were evaluated every week for 4weeks. Consumption of SE2® and StemFlo® reduced the healing time by one week while reducing the consumption of pain medication. Maximum difference in recovery was seen after 21days. Endogenous stem cell mobilization could represent an effective adjunctive approach in the treatment of sports injuries.
Keywords: se2, stemflo, stemenhance, stem cells mobilize, ankle injury, sport injury, stem cell, ligaments
BMSC, bone marrow-derived stem cells; ESCM, endogenous bone marrow stem cell mobilization; KAFS, karlsson and peterson scoring system for ankle function; GFP, green fluorescent protein
Soccer is one of the most popular sports worldwide with about 265million players, both professionals and amateurs.1 This popularity has massive financial implications especially when considering professional soccer. Extensive research has been done regarding various types of injuries, with emphasis on professional players since they have a greater exposure than soccer players at the recreational level. One of the most significant findings from the epidemiological studies of Woods et al.2,3 was the disproportionately high number of training injuries during preseason, and match injuries during the early stages of the season. Several reasons for this increased incidence have been suggested, including hard playing surface,4 high training intensity,5 sudden change in training intensity from closed season to preseason, and short preseason preparation.6
The most common injuries are muscle and ligaments sprains, with muscle sprains being the first and most common of both injuries observed during preseason.2 It is crucial to the practice of sport medicine to find new ways of reducing the healing time and improving total recovery for these types of injuries and athletes.
Injuries to the skin, bones and muscles were shown to trigger mobilization of bone marrow-derived stem cells (BMSC) and their migration into the injured tissue where they participate to the process of tissue repair.7–11 It was documented that the speed and extent of tissue repair depends in part on the number of peripheral blood stem cells available to migrate into an injured tissue.12–14 The stem cell mobilizer StemEnhance®10 has been shown in an animal study to accelerate muscle repair after induced muscle injury.15 StemEnhance® is a natural product containing an L-selectin blocker that works by interfering with the SDF-1/CXCR4 axis in the bone marrow, triggering stem cell mobilization.16 Both StemEnhance® and its advanced formula SE2® have been shown to trigger a mild increase in the number of peripheral blood stem cells, unfolding a great potential at the clinical level. StemFlo® is a dietary supplement containing fibrinolytic enzymes and antioxidants aimed at optimizing blood circulation in the fine vasculature (in preparation). This study was aimed at investigating the clinical potential of Endogenous Bone Marrow Stem Cell Mobilization (ESCM) using the stem cell mobilizer SE2® and StemFlo®, when used along with conventional treatment for sport ankle injuries, in order to decrease recovery time and reduce the critical off match time for professional soccer players.
Subjects
A total of 12 male professional soccer players from a professional team in Madrid, Spain, aged 18 to 22years old, were randomly assigned to either experimental or control group. Athletes were in general good health and free of any health problems, including neurological or systemic disorder that would interfere with the results. All participants presented the same type of injury consisting of ankle sprain Grade II with partial tearing of the ligaments, moderate pain, joint instability and swelling with bruising throughout the ankle and foot. None of the participants showed any fracture. Athletes with bilateral ankle sprain, ipsilateral knee injury, third degree sprain, and previous ankle sprain within 6months were excluded from the study.
The participants were pair-matched, considering age (20.0±1.4 vs 19.8±1.2), height (177.8±2.1vs178.3±3.1 cm), body weight (78.3±4.1 vs 79.2±3.2 kg), and extent of injury in control and experimental groups, respectively.
The Evaluations for each subject included global assessment, medical exam, soft tissue sonogram, and the Karlsson and Peterson Scoring System for Ankle Function (KAFS) for both groups at beginning of the study and each subsequent week for a total of 4weeks. The KAFS is a validated disease-specific scale (0-100) that was developed to evaluate individuals with ankle injury. This scale measures eight parameters: instability, pain, swelling, stiffness, specific motion activities and need for support. Results are classified in four categories: excellent (90-100 points), good (80-89 points), fair (60-79 points) or poor (≤60 points). Patients were evaluated by team's doctors with soft tissue sonogram at days 1, 7, 14 and 21.
Consumable and treatment
Both groups received conventional treatment for ankle injury consisting of non-steroidal anti-inflammatory medication as needed, application of a cold pack, manual therapy, complete or partial immobilization (with cast, brace, or bandage), neuromuscular training and balance training. In addition, the experimental group (SE2/SF) received 12 capsules of SE2®and 3 capsules of Stemflo® daily for 4weeks. Both SE2® and StemFlo® were provided by Stemtech International, Inc., Florida, USA. The control group did not receive a placebo.
The study was evaluated and approved by the Ethical Committee of Clinica Quantum (Spain). The protocol met all requirements established by the Conference of Helsinki for research on humans.
All 12 subjects completed the study. The overall severity of acute ankle injuries was similar in both groups at the beginning of the trial. The extent of ankle injury was identical between pairs of players at the beginning of the study.
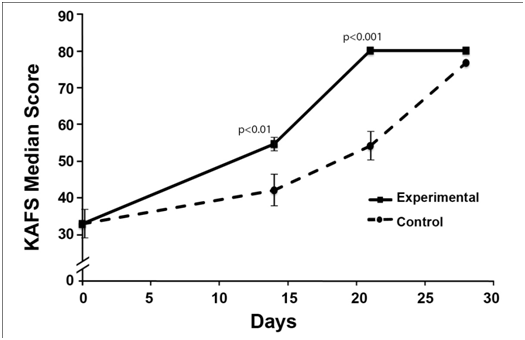
Subjects in the experimental group showed significant improvement in their physical examination and soft tissue sonogram after14 days. KAFS Analysis evidenced a noticeable difference in the course of recovery between the two groups. The SE2/SF group experienced a significantly greater reduction in pain, range of motion and joint stability associated with their ankle injury compared with control group (Figure 1). The time to heal the acute ankle injury sufficiently to go back to playing soccer was on average 17 days for the SE2/SF group compared to 21 days and over for the control group. However, on the basis of the KAFS assessment, full recovery was seen after 21 days in the SE2/SF group compared to 28 days for the control group. After 14 days the overall KAFS Median score was 89 for the experimental group and 72 for the control group, positioning the two groups in two different categories of recovery levels based on the KAFS Median score: good (80-89 points) for experimental group and fair (60-79 points) for the control group (Figure 2).
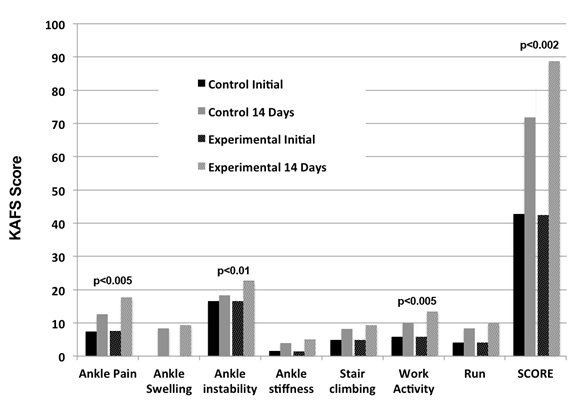
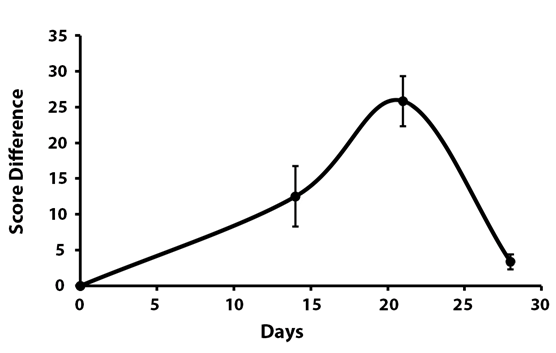
When analyzing the data using pair matching, the maximum difference in recovery between the two groups could be seen with every single parameter after 21 days (Table 1). At that time, the SE2/SF group scored 25.8 ± 3.4 above the control group in the total KAFS Median score (Figure 3).
|
Days |
|||
|---|---|---|---|---|
0 |
14 |
21 |
28 |
|
Ankle Pain |
0 ± 0 |
4.2 ± 0.8 |
4.2 ± 0.8 |
0 ± 0 |
Ankle Swelling |
0 ± 0 |
3.3 ± 1.1 |
6.7 ± 1.1 |
0 ± 0 |
Ankle Instability |
0 ± 0 |
1.7 ± 1.1 |
5 ± 0 |
0 ± 0 |
Ankle Stiffness |
0 ± 0 |
0 ± 0 |
2.5 ± 0.5 |
0 ± 0 |
Stair Climbing |
0 ± 0 |
2.5 ± 1.7 |
4.2 ± 0.8 |
0 ± 0 |
Run |
0 ± 0 |
0.8 ± 1.5 |
3.3 ± 1.1 |
-1.7 ± 1.1 |
Score |
0 ± 0 |
12.5 ± 4.2 |
25.8 ± 3.4 |
3.3 ± 1.1 |
Table 1 Difference in KAFS scores between the experimental and control groups when using the pairing of the players and comparing the extent of healing at the various time points. Maximum difference in healing was seen at 21 days
The study was intended only as a complementary protocol to expedite the recovery in athletes with this type of injuries. However, additional benefits were also revealed in the experimental group with regards to pain level. Individuals in the experimental group requested less anti-inflammatory and pain medication.
The development of protocols that not only efficiently treat sport injuries but also expedite the recovery is the keystone of the practice of sport medicine. In this scope, this study further documents the therapeutic potential of Endogenous Stem Cell Mobilization17 in tissue repair, and more specifically in sport injuries. This study documented that the use of the stem cell mobilizer SE2®along with StemFlo®, a fibrinolytic product aimed at improving blood microcirculation, in conjunction with conventional treatment reduced the recovery time from acute ankle injuries in male soccer players. Complete recovery was on average reduced by one week with the use of SE2®and StemFlo®. The time to revert the acute ankle injury was on average 21days for the SE2/SF group and 28days for the athletes in the control group. Nevertheless, players in the control group on average returned to play only 4days after the SE2/SF group (17 vs 21days), indicating that the control group might not have recovered as much as the SE2/SF group before returning to play, therefore exposing them to a greater risk of possible recurring injury. Unfortunately, post-treatment recurrent injuries were not monitored as part of this study.
Stem Enhance®, a concentrate of the cyanophyta Aphanizomenon flos-aquae that was documented to trigger bone marrow stem cell mobilization16 has been shown to significantly accelerate muscle repair after injection of cardiotoxin in the tibial is muscle of irradiated mice transplanted with bone marrow stem cells expressing green fluorescent protein (GFP).15 In this study, muscle repair took place through the migration of circulating bone marrow–derived stem cells into the injured muscle and their subsequent differentiation into muscle cells, as shown by the incorporation of GPF-positive muscle cells in the regenerating muscle. Stem Enhance® was also reported to promote tissue repair and significant improvements in single patient outcomes associated with a wide variety of health conditions linked to tissue damage or degeneration.17
Other studies have documented the healing potential of bone marrow stem cell mobilization.13 For example, Bozlar et al.18 injected the stem cell mobilizer G-CSF (25μg/kg/day) or 0.9% saline in rats that had been subjected to fracture of the tibiae. Radiological, histological and biomechanical assessment performed 3weeks later revealed a significantly greater recovery in the G-CSF treated animals. Likewise, in mice subjected to burn and incision of the skin, animals treated with G-CSF showed greater recovery.19
CD34+ stem cells collected from peripheral blood following mobilization induced by G-CSF were locally injected along with atelocollagen in rats subjected to medial collateral ligament injury, and the results were compared with a control group only treated with atelocollagen. Animals subjected to G-CSF and then injected with CD34+ stem cells showed much greater recovery, using macroscopic, histological, and biomechanical assessments.20 Greater tissue repair was at least in part due to enhanced neovascularization in the treated animals.
Endogenous Stem Cell Mobilization and more specifically the use of the stem cell mobilizer SE2® along with StemFlo® therefore emerge as possible complementary protocols to support the effectiveness of conventional treatment for sport ankle injuries, thereby decreasing the recovery time and reducing the critical off match time for the professional soccer player. Further investigations should be done with a broader spectrum of sport injuries.
None.
The author declares no conflict of interest.

©2015 Garber, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.