MOJ
eISSN: 2374-6912


Mini Review Volume 3 Issue 4
Department of Cell Biology, University of Concepción, Chile
Correspondence: María de los Ángeles García-Robles, Department of Cell Biology Laboratory of Cell Biology, Faulted of Biological Sciences, University of Concepcion, Concepcion, Chile, Tel 5641 2203805, Fax 56 41 2239687
Received: May 27, 2016 | Published: August 9, 2016
Citation: Elizondo-Vega R, Salgado M, García-Robles MA. MOJ Cell Sci Rep. MOJ Cell Sci Rep. 2016;3(4):114-117. DOI: 10.15406/mojcsr.2016.03.00066
Monocarboxylate transporters (MCTs) are a family of transporters, which participate in the facilitated diffusion of lactate as well as the incorporation and release of several other metabolically important monocarboxylates, such as Pyruvate and ketone bodies. It has been established that lactate and ketone bodies have a role in energy balance. In the brain, lactate is an important oxidative energy substrate, and its intracerebroventricular (ICV) administration decreases food intake in rats. An opposite effect is produced by the ketone bodies. Several studies have suggested that the glucosensing mechanism is governed by a metabolic interaction between neurons and glial cells via lactate flux through MCTs. Hypothalamic glial cells (tanycytes) release lactate through MCT1 and MCT4. Moreover, orexigenic and anorexigenic neurons of the arcuate nucleus (AN) have high membrane immunoreactivity for MCT2, which is involved in lactate influx, suggesting that tanycytes indirectly control neuronal activity via glucose. This review will focus on MCT expression in the hypothalamus, a master regulation center for glucose homeostasis, and the evidence for a metabolic interaction between tanycytes and neuroendocrine neurons using monocarboxylates.
Keywords:monocarboxylate, tanycytes, neuroendocrine neurons, glucose, hypothalamus, glial cells
MCT1, monocarboxylate transporter; AgRP, agouti-related peptide; NPY, neuropeptide y; POMC, pro-opiomelanocortin; ICV, intracerebroventricular; 3V, third ventricle; AN, arcuate nucleus
Monocarboxylate transporters (MCTs) have a high capacity to transport short-chain monocarboxylates, such as lactate, Pyruvate and the ketone bodies (KB), α-hydroxybutyrate and acetoacetate, which have a role in energy balance. In the brain, lactate is an important oxidative energy substrate,1 and its intracerebroventricular (ICV) administration decreases both food intake and blood glucose levels in rats.2 Ketone bodies also affect food intake3,4 and body weight in an opposing manner.5 In the hypothalamus, a brain region involved in energy balance, it has been shown that MCTs have a particular cellular distribution, which supports the transfer of monocarboxylates between glia and neurons. MCT1 and MCT4 are expressed in the main glial cells (tanycytes) in the basal hypothalamus. α and β-tanycytes are specialized ependymal cells that bridge the cerebrospinal fluid (CSF) and neuroendocrine neurons localized in the arcuate nucleus (AN)6 (Figure 1). Specifically, tanycyte processes that are MCT1- and MCT4-positive are in direct contact with MCT2 positives neurons that synthesize and release neuropeptides that control food intake, such as neuropeptide Y (NPY)-, agouti-related protein (AgRP)-, and proopiomelanocortin (POMC).7,8 MCTs comprise a family of fourteen members (MCT1-14) distributed in a wide variety of tissues;9–11 they use the electrochemical gradient of protons to translocate monocarboxylates and protons at a 1:1 ratio in the same direction.12,13 MCT1 has the widest selectivity, and may transport several short chain fatty acids, such as acetate, butyrate, lactate or Pyruvate.14 Brain localization studies using immunocytochemistry, in situ hybridization and Immunoblotting have determined that MCT1 is predominantly a glial transporter, expressed in cortical astrocytes,15–22 hippocampal astrocytes,23,24 astrocytes of the supraoptic nucleus,21 choroid plexus, ependymal ciliated cells,22,25 endothelial cells and pericytes.17,20 In vitro neuronal expression of MCT1 has also been reported and recently in NPY-expressing neurons in situ.3 MCT2 has a much higher affinity for Pyruvate although it also transports lactate and ketone bodies.14. MCT2 localization is mainly at the neuronal level in the cortical neurons,26 and cerebellar Purkinje cells,27 in the postsynaptic density of the asymmetrical excitatory synapses of the hippocampus28 Purkinje neuron cell bodies.27 In addition, MCT2 was detected in marginal glia, ependymal cells18 and the vascular processes of astrocytes.18,22 Recently, we have identified MCT2 localization in anorexigenic and orexigenic neurons of the AN.7 In accordance with a participation in energy balance, it has been reported that insulin stimulates the expression of MCT2 in cultured neurons.29 MCT3 expression has only been characterized using Northern blot and immunohistochemistry studies and found in the basolateral membrane of the choroid plexus.30 MCT4, which transports lactate and ketone bodies and has a very low affinity for Pyruvate, was detected exclusively in glial cells, particularly in the Bergmann glia and astrocytes of the molecular and granular layers of the cerebellum.21,24,27,31 In addition, MCT4 expression was detected in astrocytes of the hippocampus, corpus callosum and cerebral cortex24 as well as in ciliated ependymal cells of the paraventricular nucleus.22 We have described the localization of MCT4 in tanycyte processes that contact distal neurons in the AN.6 Therefore, the presence of MCT1 and MCT4 in tanycytic basal processes and MCT2 expression in AN neurons suggest that lactate could be transferred between both cellular types as a signal derived from glucose for regulating energy balance and food intake. In support of this model, it has been demonstrated that glucose increases intracellular Ca2+ concentration ([Ca2+]i) in cultured tanycytes, a response that is oxidative phosphorylation-independent but can be diminished through the inhibition of glucokinase, a critical glucosensor enzyme expressed in tanycytes.32–34 Interestingly, this mechanism involves the opening of hemi channels (HC), and pharmacological inhibitors of HC abolish the response to glucose, but do not affect the efflux of lactate.6,33,35 Several studies have shown that ICV administration of lactate decreases food intake and stimulates pancreatic insulin secretion.36,37 Moreover, recent studies demonstrate that glial connexins are required for hypothalamic glucosensing, suggesting that glial glucose metabolism is needed for centrally mediated glucose-induced insulin secretion as well as suppression of food intake.36 Brain use of ketone bodies in normoglycemia is very low, however in fasting or high-fat diets this increases considerably.4 Ketone bodies, mainly produced in liver, are incorporated into the brain by MCT1, which is expressed in endothelial cells of cerebral blood vessels.38–40 Interestingly, the hypothalamus, which is a key player in brain sensing of metabolic signals, presents higher ketone body metabolism than other brain areas (Figure 2).41 Furthermore, it has been shown that astrocytes in culture could release ketone bodies, generated by the catabolism of fatty acids.42 It is also possible that ketone bodies can be generated by glial cells of the hypothalamus (astrocytes or tanycytes). In support of this notion, the presence of numerous lipid droplets and the expression of enzymes involved in lipid metabolism have been demonstrated in tanycytes using histological and immunohistochemical analyses.43 Therefore ketone bodies release could be realised by glial cells and/or peripheral by hepatocytes. Alternatively, the neuronal effects of ketone bodies could be mediated by MCTs or through hydroxy-carboxylic acid receptors, which have been located in several brain regions44 and which are Gi-coupled receptors.45–47 The mechanisms that allow to ketone bodies to modulate the activity of neurons that control food intake still remain to be elucidated.
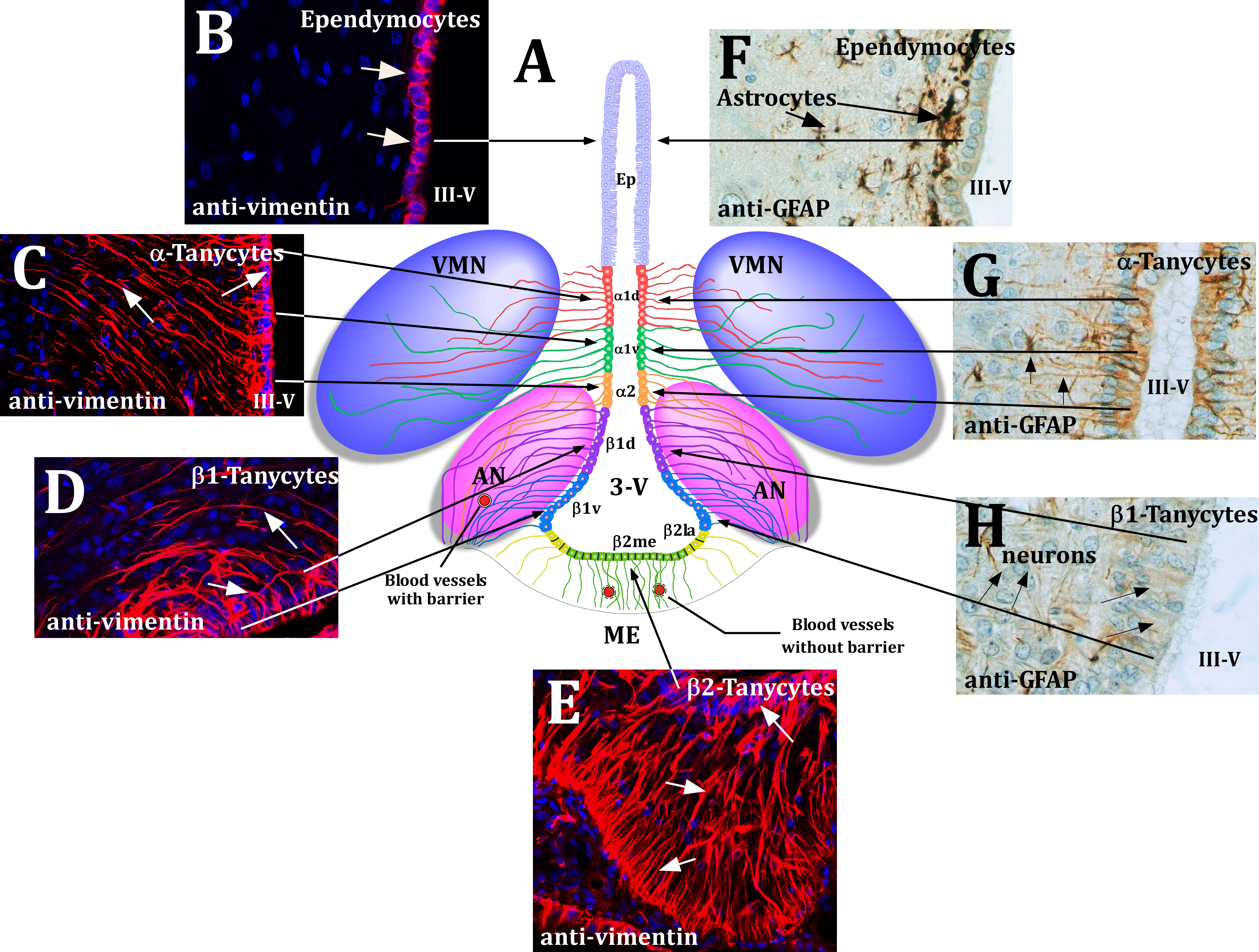
Figure 1 Schematic representation of the hypothalamus
(A) Frontal view of the hypothalamus.
1. Ciliated Ependymocytes;
2. α1 and α2-tanycytes contacting neurons of the AN, VMN and blood vessels;
3. β1-tanycytes line the infundibular recess, and their basal projections reach the lateral regions of the ME and the AN;
4. β2-tanycytes cover the floor of the III-V and extend their projections inside the ME.
(B-E) Immunohistochemical analysis of the hypothalamic tanycytes. Representative confocal images of frontal hypothalamic sections immunostained with anti-vimentin antibody. Ependymocyte cells (B), a (C), β1 (D) β2-tanycytes (E) are positive for the expression of vimentin.
(F-H) Immunohistochemical analysis of hypothalamic glial cells. The hypothalamic sections were incubated with anti-GFAP and the immunoreaction was developed using the PAP method. Astrocytes, localized under the ependimary cells (F) and α-tanycytes (G) are positive for GFAP. A weaker reaction was found in β1-Tanycytes (H).
AN: Arcuate Nucleus; VMN: Ventromedial Nucleus; Ep: Ependymocytes; 3-V: Third Ventricle.
Modificated from Elizondo-Vega et al.8
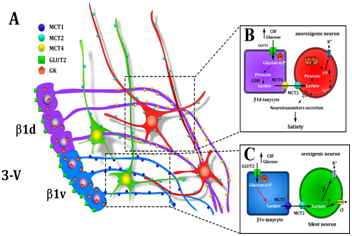
Figure 2 Model of cerebral glucose sensing based on the metabolic interaction between β1d-tanycytes or β1v-tanycytes and neurons.
(A) Schematic representation of the location of MCT4 (yellow) in β1d-tanycytes processes (purple), MCT1 (blue) in β1v-tanycytes processes (light blue), and MCT2 (light green) in orexigenic (green) and anorexigenic (red) neurons of the AN.
(B) Schematic overview of the model of glial-neuronal interaction based on the transfer of lactate proposed for cerebral glucose sensing between GE neurons and tanycytes.
(C) Scheme based on proposed interaction between β1v-tanycytes and orexigenic neurons compared to the increase in glucose concentration in the CSF. 3-V: Third Ventricle; Β1d and Β1v: Tanycytes; CSF: Cerebral Spinal Fluid; GK: Glucokinase; LDH: Lactate Dehydrogenase. Modificated from Elizondo-Vega et al.8
MCTs mediate the release of lactate from tanycytes and its incorporation into neurons, which could generate satiety when glucose concentrations rise. In contrast, fasting could favor the incorporation of ketone bodies through the blood-brain barrier or their release from glial cells, activating neurons that induce hunger or inhibiting satiety neurons. Current literature supports the participation of MCTs in food intake, which is relevant under low- and high-glucose conditions.
None.
The author declares no conflict of interest.

©2016 Elizondo-Vega, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.