MOJ
eISSN: 2374-6912


Research Article Volume 2 Issue 2
1Research Institute of Orthopedics, Xiaoshan Traditional Chinese Medical Hospital, China
2Research Institute of Orthopedics, Zhejiang Chinese Medical University, China
Correspondence: Renfu Quan, Research Institute of Orthopedics, Xiaoshan Traditional Chinese Medical Hospital, ZhengJiang Province 311200, China, Tel 8657183812008
Received: March 15, 2015 | Published: April 17, 2015
Citation: Quan R, Zheng X, Xie S, et al. Lentivirus-mediated expression of VEGF 165 in rat hair follicle stem cells. MOJ Cell Sci Rep. 2015;2(2):31-36. DOI: 10.15406/mojcsr.2015.02.00022
Tissue-engineered skin is the latest most effective treatment for major skin defects, whereas the difficulty of vascularization prevents further development problematic. The purpose of our study was to transfect rat hair follicle stem cells (rHFSCs) with vascular endothelial growth factor-165 (VEGF165) using lentivirus vectors. rHFSCs were harvested from 1-week-old Sprague-Dawly (SD) rats and digested with dispase and collagenase IV. rHFSCs were cultured and their growth curve was plotted. They were then characterized by detecting marker genes with immunofluorescent staining and real-time polymerase chain reaction (RT-PCR). Transfection was performed using either pLV-VEGF165-IRES (internal ribosome entry site)-EGFP (enhanced green fluorescent protein) (experimental group) or pLV-IRES-EGFP (blank control). Expression of VEGF165 mRNA and protein were detected with RT-PCR and western blotting, respectively. The cultured rHFSCs showed typical cobblestone morphology with good proliferative capacity. VEGF165 was successfully transfected into the cells at 2weeks at a rate of 85.8±1.9%. Both VEGF165 mRNA and protein were examined in the experimental cells. Therefore rHFSCs can be successfully and effectively isolated with high purity and good proliferative capacity. rHFSCs transfected using lentivirus expressed high levels of VEGF165, so are potentially an ideal cell source for tissue-engineered hair follicles, vessels and skin.
Keywords: tissue engineering, hair follicle stem cells, vegf165, lentivirus, gene modification, rats
rHFSCs, rat hair follicle stem cells; VEGF165, vascular endothelial growth factor-165; SD, sprague-dawley; RT-PCR, real-time polymerase chain reaction; IRES, internal ribosome entry site; EGFP, enhanced green fluorescent protein; HFSCs, hair follicle stem cells; VEGF, vascular endothelial growth factor; DMEM, dulbecco’s modified eagle’s medium; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; CK15, cytokeratin 15; DAPI, 4,6-diamidino-2-phenylindole; KSR, knockoutTM serum replacement; P2, second passage; P3, third passage; PBST, phosphate buffered saline tween; ESCs, embryonic stem cells; MSCs, mesenchymal stem cells; ADSCs, adipose-derived stem cells
Large skin defects caused by trauma often lead to serious physical disability and even death. Treatments for large skin defects include wound dressings, autologous skin grafts, allogeneic skin grafts and tissue-engineered skin. However, issues arise when using these methods that lessen their efficacy. Wound dressings have no physiological function. Autologous skin grafts are limited by source. Allogeneic skin grafts can result in transplant rejection, skin necrosis and secondary damage to patients. Tissue-engineered skin is often accompanied by wound infection, nonunion and other complications, predominantly caused by poor vascularization of the wound surface.1,2 Poor vascularity is the primary reason for necrosis and graft failure of tissue-engineered skin. Therefore, vascularization is one of the major challenges currently facing tissue-engineered skin.3–5
Hair follicle stem cells (HFSCs) are undifferentiated cells with self-renewing and good proliferative properties. These cells are primarily found in the bulge of the outer root sheath of hair follicles.6,7 In vitro studies showed that HFSCs have high cloning capacity with a good regenerative potential.8 HFSCs can not only differentiate into hair follicles, but can also differentiate into nerve, melanoma, smooth muscle and epithelial cells. HFSCs are derived from skin and hair follicles. Therefore, an abundant source of autologous HFSCs can be easily isolated from patients. No serious complications have been reported during the harvesting of HFSCs, and these are one of the most easily obtained stem cells.9,10 Vascular endothelial growth factor (VEGF) is a vascular endothelial cell-specific mitogen that is involved in angiogenesis and tissue repair. VEGF165 is one of the 5 VEGF subtypes, and is also the most potent and widely distributed.11 Lentivirus vector can effectively transfect non-dividing cells with large exogenous gene fragments. Lentivirus also has a low immune response and can be modified many times with a good safety profile.12,13 We therefore transfected rat HFSCs (rHFSCs) with VEGF165 using lentivirus vectors. These cells were examined for target gene expression. rHFSCs expressing VEGF165 can improve vascularization of tissue-engineered skin.
Animals, reagents and instruments
Six 6-week old SD rats, male and female, weighing 25±2g, were purchased from the Shanghai Slack Laboratory Animal Limited Liability Company (Shanghai, China, Certificate number: 2007000540183). pLV-IRES-EGFP and pLV-VEGF165-IRES-EGFP plasmids were kindly given by Dr. Jianfeng Chang of the College of Life Science and Technology, Tongji University.
Dulbecco’s modified Eagle’s medium (DMEM)/F12 (1:1) medium, high glucose DMEM medium, Knockout Serum Replacement, collagenase IV, dispase, Coating Matrix (50×), TrypLETM Express alternative enzyme trypsin (1×), L-glutamine, non-essential amino acids, and hydroxyethyl alcohol were obtained from (GIBCO, USA). Other reagents included, recombinant human epidermal growth factor (EGF), recombinant human Basic fibroblast growth factor (bFGF) (R&D, USA), penicillin-streptomycin mixture (Suolaibao, Beijing, China), hydrocortisone (Sangon Biotech, Shanghai, China), aqueous mounting medium (Kangwei Century Biotech, Beijin, China), collagen IV (BD, USA), Trizol RNA extraction reagent, reverse transcriptase, Taq DNA polymerase (Invitrogen, USA), real-time PCR reaction kit (ABI, USA), polyclonal antibodies against integrin β1, cytokeratin 15 (CK15; Abcam, USA), integrin α6 (Santa Cruz, USA), rabbit anti-mouse IgG (Jackson, USA), 4,6-diamidino-2-phenylindole (DAPI) (Roche, Switzerland), Lenti-X lentiviral expression system (with 3 packaging plasmids VSVG, RSV-REV and RRE; Clontech, USA), 293T cells (provided by Dr. Guoping Fan of the Tongji University Stem cell Center) and a calcium phosphate cell transfection kit (Baienwei Biotech, Shenzhen, China). Instruments used included a fluorescent microscope, inverted contrast phase microscope (Olympus, Japan), real-time PCR instrument, electrophoresis, gel imaging system (Bio-Rad, USA), and Odyssey machine (LI-COR, USA).
Isolation, culture and purification of rHFSCs
rHFSCs were isolated and cultured according to previously described methods.14,15 In brief, one-week old SD rats were sacrificed by cervical dislocation and disinfected in 75% ethanol. The skin near the beards was cut into 2×2mm blocks using ophthalmic scissors and rinsed 3times with PBS. Tissue blocks were treated with 1% dispase and 1% collagenase IV at 37°C for 90min, and washed 3times with PBS. Under a stereomicroscope, the hair follicle was separated from the connective tissue sheath using a syringe needle. The 2 ends were cut off, leaving the bulges, which were cultured in BD, USA) with 1mL complete medium (869μL/mL DMEM/F12, 100μL/mL KnockOutTM Serum Replacement (KSR), 10μL/mL penicillin-streptomycin mixture, 10μL/mL L-glutamine, 10μl/mL non-essential amino acids, 20ng/mL EGF, 10ng/mL bFGF, 1μL/mL hydroxyethyl alcohol, 10ng/mL hydrocortisone) at 37°C in air with 5% CO2 for 2 days. After tissue adherence, 5mL complete medium was slowly added, which was changed every 3days. Cell migration and growth were followed microscopically. HFSCs were digested with TrypLETM Express alternative enzyme trypsin at 37°C for 8min before passage.
Collagen IV-coated dishes were maintained at room temperature for 1h. Primary cells were digested from follicle bulges with TrypLETM Express alternative enzyme trypsin at 37°C for 8min and centrifuged at 500g for 5min. Cells were pipetted to form a single-cell suspension and cultured on the collagen IV-coated dishes for 20min. Non-adherent cells were discarded with the culture medium. Adherent cells were further cultured in complete medium, with a medium change every 3days. Primary cells and second passage (P2) cells were purified using this same method. Cell morphology was observed microscopically.
Real-time polymerase chain reaction (RT-PCR)
Third passage (P3) rHFSCs were used for RT-PCR. The target genes, CK10, CK15, CK19, CD34, integrin β1, integrin α6 and reference gene ACTB were amplified in different reaction tubes. The primers were designed by Primier 5.0 software (Table 1). The PCR reaction system comprised 2×SYBR Green Mix (10μL), primer Mix (1μL), template (1μL), and H2O (8μL). Reaction solutions were then loaded into AXYGEN PCR tubes centrifuged briefly and inserted into the real-time PCR instrument. The SYBR Green method was used and the melting curve was set to start from 80°C. The relative mRNA expression level of each gene was calculated using the -ΔΔCt method.
Immunofluorescent staining
P3 rHSFCs cultured to the logarithmic phase were digested, centrifuged, and cultured on chamber slides at 1×105cells/well for 2days. The culture medium was discarded and cells were washed with and fixed with 4% paraformaldehyde. Bovine serum albumin (5%) was added at room temperature. Primary antibodies against integrin β1 (1:100), integrin α6 (1:50) and CK15 (1:100) were added separately to cell samples at room temperature, with a negative control carried out in the absence of the primary antibody. After washing with PBST, TRITC-labeled rabbit anti-mouse immunoglobulins was added and incubated for 30min in the dark followed by DAPI addition (1:2000) and further incubation for 5min to counter stain the nuclei. The slides were dried in the dark and mounted with water-based mounting medium. Red fluorescence was examined under a fluorescence microscope.
Cell growth curve
rHFSCs (P3, P5, P7 and P9) were digested at 37°C for 8min prior to seeding in 24-well plates at 1×105cells/well. Cells were counted on days 1, 2, 3, 4, 5, 6, and 7. Six wells were counted for each time-point and the mean cell number was calculated to plot growth curve.
Lentivirus packaging
Lentivirus was packaged with a calcium phosphate transfection kit. 293T cells in logarithmic growth phase were cultured in 10mm dishes for 24h until 50-70% confluence. Before transfection, medium was replaced with H-DMEM containing 10% FBS. The target plasmid pLV-VEGF165-IRES-EGFP and 3 packaging plasmids (VSVG, RSV-REV and RRE) were gently mixed in HBS buffer at a ratio of 2:1:1:1. ddH2O was added to prepare solution A. CaCl2 solution was added to solution A and placed at room temperature for 20min. Solution A was added drop-wise to the cell culture dish, which was horizontally shaken. Cells were incubated at 37°C in air with 5% CO2 for 10-12h and the medium replaced with H-DMEM containing 1% penicillin-streptomycin and 10% Fetal Bovine Serum (FBS). After 48h, the cells had grown to confluence and showed strong green fluorescence. Culture supernatants were collected, filtered through a 0.45μm membrane filter and centrifuged at 55,000g at 16°C for 3h. Supernatants were discarded and complete medium added to the precipitates. The medium was pipetted, divided into 2 tubes and stored at -80°C. pLV-IRES-EGFP plasmid vector packaging was treated in the same way and used as a control.
Lentivirus transfection
P3 rHFSCs were digested at 37°C for 8min and centrifuged at 500g for 5min. Virus solutions (50µL) and complete medium (50μL) were added. Cells were pipette and seeded into 24-well plates pre-coated with Matrix gel before being placed at 37°C with 5% CO2 for 30min. Complete medium was then added for further culturing after 24h.
EGFP expression
rHFSCs were cultured for 72h and then observed under a fluorescence microscope. Green fluorescence confirmed EGFP expression. Twelve fields of view (200× magnification) were randomly selected to calculate the transfection efficiency using the formula: number of positive cells / total cell number in the field × 100%. The average was taken from 3 repeated measurements. Cells were cultured for a further 14days and the calculation was repeated.
RT-PCR
rHFSCs were cultured for 7days and used for detection ofVEGF165 expression by RT-PCR. Total RNA was extracted using the Trizol RNA extraction kit. cDNA was obtained with reverse transcriptase. The primers were designed using Primier 5.0 software (Table 1). The PCR reaction system comprised cDNA template (1μL), 10× PCR buffer (2μL), Taq DNA polymerase (1U), primers (1μL), and H2O added up to 20μL. The PCR program was: 5min at 94°C, 1min at 95°C, 1min at 58°C, 1min at 72°C, for 35 cycles, with a final 10min at 72°C. Product (5µL) was separated in 2% agarose gel for 30min and visualized using the Bio-Rad imaging system.
Gene |
Primer Sequences (5’→3’) |
|
|---|---|---|
|
Forward |
Reverse |
CK10 |
TTGGAAACCTGCAAATAACCC |
ATCATAGACGAAAGGACTCTACCC |
CK15 |
AAAACCGTCGGGATGTAGAGG |
TTGCTGGTCTGGATCATTTCTGT |
CK19 |
CCAAGTTTGAGACAGAACAGGC |
CGTGGTTCTTCTTCAGGTAGGC |
CD34 |
CCTGCCGTCTGTCAATGTTTC |
GCACTCCTCGGATTCCTGAAC |
Integrin β1 |
ATCATGCAGGTTGCAGTTTG |
CGTGGAAAACACCAGCAGT |
Integrin α6 |
CGTGGTTCTTCTTCAGGTAGGC |
CACATCTATGGACGCCCTCAC |
ACTB |
GCTATGTTGCCCTAGACTTCGA |
GATGCCACAGGATTCCATACC |
VEGF165 |
CACCCACCCACATACATACA |
CTCCCAACTCAAGTCCACA |
β-actin |
GTCCCTCACCCTCCCAAAAG |
GCTGCCTCAACACCTCAACCC |
Table 1 Sequences of primers
Western blotting
7days after transfection, VEGF165 protein expression was examined by western blotting. Cells were lysed on ice and total protein was extracted. The sample was centrifuged at 4000g at 4°C for 5min. The supernatants were run on an SDS-PAGE gel before transfer onto a PVDF membrane. The membrane was soaked in 5% skimmed milk for 1h and transferred into blocking solution overnight. It was washed 3times with PBS and exposed to primary antibody against VEGF165 (1:2000) for 1h. The membrane was washed 3times with TBST, prior exposure to rabbit anti-goat secondary antibody (1:1000) at room temperature for 1h. It was washed 3times with TBST, prior to adding ECL reagent. Bands were visualized and photographed by the Odyssey machine.
Morphology of rHFSCs
On day 3 of hair follicle culture, some cells had migrated out from the hair follicle bulge (Figure 1A). On day 7, the cell number had increased, surrounding the hair follicle bulge (Figure 1B). On day 14, ~60% of the cells showed cobblestone morphology and were closely aligned, typically of epithelial cells. The remaining cells were round or spindle-shaped and had firmly adhered to the dish (Figure 1C). Following cell purification by culture on collagen IV (at primary and second passage), ~95% showed typical stem cell morphology. These characteristics included cobblestone and nest appearances, a stereoscopic impression, high refractive index, high colony-forming ability, small cell size, and round and large nuclei that were centralized in the cell (Figure 1D & 1E).
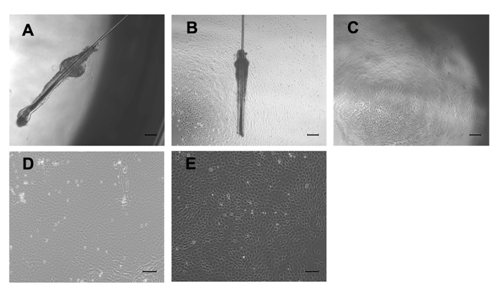
Figure 1 Morphological observation of rHFSCs by inverted phase contrast microscope.
A: A few cells climbed out from the surrounding of the hair follicle bulge after 3 days in culture; B: Cell number increased gradually after 7 days; C: Primary cells cultured for 14 days; D: Primary cells after purified once; E: The 2nd generation cells after one round of purification; Bars: A-C: 200μm; D,E: 100μm
rHFSC gene expression
HFSC marker genes such as CK15, CK19, integrin β1, and integrin α6 were highly expressed at mRNA level, with levels relative to b-actin being -9.91±0.23, -5.42± 0.05, -6.97± 0.37, and -7.14 ± 0.31, respectively. mRNA expression of keratinocyte markers, CK10, an epidermal stem cell marker, CD34, were relatively low (-23.25 ± 0.26 and -15.81±0.13, respectively).
Immunofluorescent staining of cells after 3 generations confirmed CK15, integrin β1, and integrin α6 expression in the cytoplasm (Figure 2), as no fluorescence was observed in the absence of the primary antibodies.
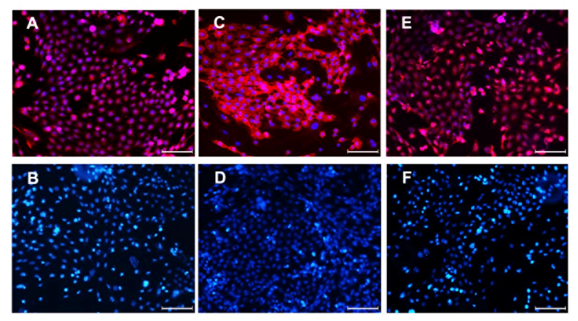
Figure 2 Immunofluorescent staining of the 3rd generation of rHFSCs (Bars: 100μm).
A: CK15; B: CK15-negative control; C: Integrin α6; D: Integrin α6-negative control; E: Integrin β1; F: Integrin β1-negative control
Cell proliferation
rHFSCs at P3, P5, P7 and P9 were in the latent phase and proliferated slowly on day 1 and 2. Stem cell clones appeared on day 3 and 4. On day 5 and 6, cells were in the logarithmic growth phase finally reaching plateau. P7 and P9 rHFSCs proliferated more slowly than P3 and P5 cells, but they proliferated faster after further passages (Figure 3).
Genetic modification of rHFSCs using lentivirus
EGFP expression: Strong green fluorescence was seen 72 h after transfection in the experimental group (Figure 4), with a transfection efficiency of 71.5±1.8%. On day 14, the lentiviral expression was steadily maintained in the cells (85.8±1.9%).
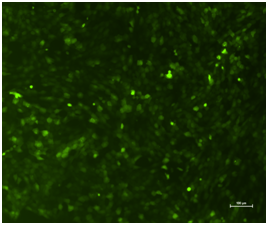
Figure 4 Inverted fluorescent microscope observation of the rHFSCs in experimental group (Bars: 100μm).
RT-PCR and western blotting: Both VEGF165 mRNA and protein were expressed in the experimental group, with negligible expression in the control group (Figure 5).
Cell source is a major challenge in the development of tissue-engineered skin, most commonly using stem cells, e.g. embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), epidermal stem cells, adipose-derived stem cells (ADSCs) and HFSCs. ESCs are totipotent in differentiation, but are severely limited by ethical issues regarding their source.16 MSCs can easily be isolated and cultured in vitro, have good proliferative properties and a low risk of immune rejection. In the microenvironment of the skin wound, MSCs can differentiate into epidermal cells and fibroblasts. However, bone marrow aspiration is needed to obtain MSCs, causing considerable trauma. Furthermore, a single bone marrow aspiration yields relative few cells, which is another limiting factor in the application of MSCs for tissue-engineered skin.17,18 Although epidermal stem cells can develop into complete skin with hair follicles and sweat glands, these cells also create many problems, such as injury during harvest and immune rejection.19 ADSCs are easily obtained, and have self-renew ability, with multiline age differentiation potential. However, the induction conditions for ADSCs need to be optimized; directed differentiation of these cells has rarely been reported.20 HFSCs have good proliferative properties and differentiation potential into epidermal and related cells. HFSCs are also rich in source and easy to obtain with minimal trauma; autologous HFSCs are free from immune rejection. HFSCs may therefore be an excellent cell source for tissue-engineered skin. In our study, using the differential adhesion method along with microdissection, dispase and collagenase IV digestion, matrix coating, the real-time PCR and immunofluorescence staining, we can successfully obtain HFSCs with good proliferative properties and purity. Then these cells were used to be transfected with lentivirus.
Conventional genetic modification techniques include the use of calcium phosphate, artificial liposomes, virus-mediation and physical methods. We found that transfection of rHFSCs using common methods, such as liposomes and calcium phosphate, were inefficient. The lentiviral vector system can achieve stable expression of target genes compared with other transfection methods.21 As carriers of small interfering RNA, lentiviral expression vector III was guided by the RNA polymerase promoter to direct the synthesis of RNA. RNA polymerase III has clear initiation and termination sequences, and this synthetic RNA has no poly-A tail. The lentiviral vector can be integrated in the genome of the target cells with good repeatability and high transfection efficiency. Lentiviral transfection does not require reagents, therefore avoiding the effects of the screening reagents on the target cells. This transfection system is highly efficient and stable,22 which is why we used lentivirus to transfect rHFSCs to get efficient and stable expression of VEGF165.
With the rapid development of genetic modification technology, transfecting target cells with specific genes and obtaining specific biological characteristics are becoming heavily researched, with VEGF gene transfection being of particular interest. The VEGF gene can be split at transcription level, producing 5 subtypes. VEGF165 is the most potent and widely distributed subtype, with many important functions. However, the half-life of VEGF165 is very short. Injected VEGF165 is rapidly diluted, with a half-life of a few hours or less23 found that the biological half-life of VEGF165 under normal oxygen partial pressure is 30-45min, but 6-8h under hypoxia conditions. Therefore, adding VEGF165 to tissue-engineered skin has very few effects. To overcome the shortcomings of protein treatment, we considered gene therapy. The abovementioned limitations can be solved using lentivirus transfection. In our experiments, we successfully use lentivirus genetic modification method to transfect rHFSCs. VEGF165 was successfully transfected into the cells at 2weeks at a rate of 85.8±1.9%. Both VEGF165 mRNA and protein were detected in the cells transfected with pLV-VEGF165-IRES-EGFP. 3 weeks after transfecting HFSCs with VEGF165 using lentivirus, we saw no typical morphological changes, such as triangular endothelial progenitor cells or spindle-shaped cells. VEGF165 expression at both protein and mRNA level was up regulated, which suggests that genetically modified HFSCs must be further investigation to confirm their ability regarding directed differentiation ability and promotion of vascularization in tissue-engineered skin.
In future studies focusing on the directed differentiation of HFSCs, these cells will be seeded in three dimensional scaffolds to build a skin substitute, which will be transplanted on to the skin of nude mice. Vascularization and wound healing processes can then be examined histologically, morphologically and immunologically. These investigations should address the problems currently facing the development of artificial skin, which presently can only partially restore the anatomical structure and physiological function of normal skin. We believe the technique we have described can become a new approach to improving the survival of tissue-engineered skin.
rHFSCs can be successfully isolated with high purity and good proliferative properties. rHFSCs transfected using lentivirus expressed high levels of VEGF165, making them potentially an excellent source for tissue-engineered hair follicles, vessels and skin.
None.
The author declares no conflict of interest.

©2015 Quan, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.