MOJ
eISSN: 2374-6912


Mini Review Volume 3 Issue 3
1Department of Regenerative Medicine, Soonchunhyang University, South Korea
2Department of Physiology, Soonchunhyang University, South Korea
3Institute of Tissue Regeneration, Soonchunhyang University, South Korea
Correspondence: Reiza Ventura, Department of Regenerative Medicine, College of Medicine, Soonchunhyang University, South Korea
Received: February 23, 2016 | Published: April 28, 2016
Citation: Ventura R, Padalhin A, Young-Ki M, et al. Bone regeneration of decellularized in-vivo deposited extracellular matrix (ECM) on hydroxyapatite sponge scaffold. MOJ Cell Sci Rep. 2016;3(3):72-74. DOI: 10.15406/mojcsr.2016.03.00057
Large bone defects and bone fractures do not heal spontaneously and considered to be one of the most challenging cases in bone reconstruction. For many fractures and bone defects, the use of hardware fixation alone is not enough to ensure fracture healing. Use of autograft, allogenic and xenegeneic scaffolds have some limitations and complications such as donor site pain and limited availability of different sizes and shapes making large defects impractical to treat.1 Different approach in tissue engineering were developed and studied to address this problem with the use of hydroxyapatite, a widely used calcium phosphate in biomedical applications, due to its close chemical composition to bones and teeth and has an excellent properties such as biocompatibility and osteoconductivity but lack intrinsic osteogenic potential found in autografts.2 The ECM is a complicated complex of many kinds of polysaccharides and proteins, such as collagens, proteoglycans and glycoproteins that are essential in tissue engineering.3 Cellular interactions with the ECM are known to play a critical role in providing structural support to cells, directing cell function, and regulating development, homeostasis, and repair of a variety of tissues.4–8 ECM derived and ECM mimicking scaffolds have shown promising results in facilitating the constructive remodeling of many different tissues in preclinical and applications.3,9,10 To obtain an ECM derived or ECM mimicking scaffolds, decellularization process were employed in native tissues and organs to remove the cellular and nuclear contents while maintaining the important proteins present in the ECM. Studies have been focus on the generation of cell derived ECM for different tissue engineering applications.11–13 However, different factors need to be considered in generation of cell derived ECM such as type of cells and the manner in which cell are cultured that involves the use of appropriate media, addition of growth factors, bioreactor design and flow rate.14 The aim of this study is to determine the feasibility and effect of in-vivo deposited extracellular matrix on bone regeneration capacity of porous hydroxyapatite sponge scaffold.
In our study,15 decellularized in-vivo deposited extracellular matrix could enhance the bone regeneration properties of hydroxyapatite (HA) scaffolds. HA sponge scaffolds were implanted in subcutaneous pockets of rats to generate ECM and extracted after 1, 2 and 3weeks (Figure 1). Decellularization treatment of in-vivo deposited HA scaffolds were employed with the use of 1% SDS, agitation and sonication to facilitate effective removal of cells. DNA and RNA content of the in-vivo deposited ECM were significantly removed after decellularization with preserved major components. DNA and RNA quantification showed decellularized in-vivo deposited ECM HA scaffolds have met the requirements Crapo et.al reported that decellularized ECM should have a DNA content of <50ng / mg dried ECM to avoid immune reaction.16 In addition, several studies reported that DNA component directly correlated with adverse host reaction upon in-vivo implantation or introduction of new cell line.16–20 ECM is composed of wide array of ECM components such as collagen, a major ECM component that provides mechanical strength and promotes cell adhesion, sulfated glycosaminoglycans (sGAG) which bind growth factors, promotes water retention and contributes to the gel properties of ECM and proteoglycans that serve as the integral membrane protein that modulates growth and proliferation of cells as well as growth factors.21,22 Retention ECM components while thorough removal of cellular and nuclear components are the goals of decellularization. Decellularization treatment removed 73% of collagen content in the ECM due to agitation and sonication that lead to significant reduction of mechanical strength, increased in porosity and pore size distribution of all decellularized scaffolds. However, 57% of sulfated glycosaminoglycan were still present in decellularized ECM as well as glycoproteins as revealed by PAS staining indicating that important ECM components were not thoroughly removed after decellularization (Figure 2). In-vitro studies revealed decellularized scaffolds have no cytotoxic effect in MC3T3-E1 pre-osteoblast cells and increased proliferation which could be attributed to presence of collagen, sGAGs and glycoproteins present in decellularized samples. These biomolecules have been found to promote early adhesion and even to contribute to differentiation.23
Based on the characterization of decellularized scaffolds, 2week in-vivo deposited ECM (D2) were chosen for in-vivo studies because it has an even distribution of ECM, a minimum pore size (75-150µm) for bone regeneration, highest compressive strength (2.05±0.78MPa), highest content of collagen (253.98±3.18µg/scaffold) and sGAGs (5.92±0.94µg/scaffold) among the decellularized samples. Bone regeneration potential and biocompatibility of an in vivo deposited ECM in HA scaffolds were evaluated by implanting D2 scaffold in 11mm critical radial defect of rabbit for 4 and 8weeks with bare HA scaffold as a control. Based on results, no hosts’ immune reaction was observed after 4 and 8weeks of in-vivo implantation period due to negligible amount of DNA (2.06±0.09µg/scaffold) and RNA (0.001±0.002µg/scaffold) content present in decellularized scaffold. Although statistical analysis of the micro CT data showed no significant differences between the bare and decellularized scaffolds, the stained tissue sections showed bare implants developed new bone localized along the implant interface while decellularized HA scaffold showed extensive network of bone/cartilage tissue complex that extends from both ends of the implant interface towards the central region. In addition decellularized groups exhibited enhanced bone fixation of implanted material with the host bone compared with bare groups after 8weeks of in-vivo implantation (Figure 3).
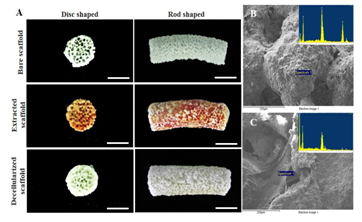
Figure 1 Images of bare, extracted, and decellularized scaffolds in the form of 5-mm discs for in vitro study and rod shaped for in-vivo study. Reproduced from Ventura et al.15
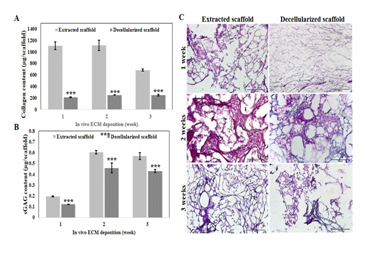
Figure 2 Evaluation of ECM content before and after decellularization. Collagen quantification
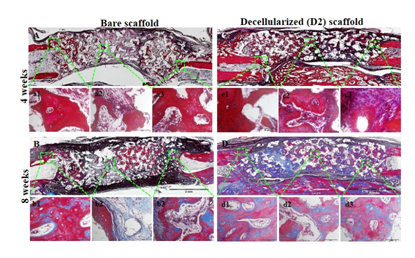
Figure 3 Masson’s Trichrome-stained sections of bare (A, B) and decellularized scaffolds(C, D) implanted for 4 and 8 weeks. Scale bar indicates 2 mm and 50 mm. Reproduced from Ventura et al.15
In summary, this study showed the feasibility of incorporating in-vivodeposited ECM on hydroxyapatite scaffolds with the use of an animal as a bioreactor. In-vivo deposited ECM in HA scaffolds are cheaper and faster compared with conventional in-vitro or cell derived method. Thorough removal of cellular materials while maintaining functional proteins present in the in-vivo deposited ECM provided an osteoconductive and osteoinductive scaffolds suitable for bone regeneration applications. Decellularized in-vivo deposited ECM on HA scaffolds provides physical support for in-vitro cell attachment and proliferation and enhanced bone regeneration and could be a potential scaffold in bone regeneration applications.
This article was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015R1A6A1A03032522). Authors would like to thank Mr. Shin-Woo Kim for his assistance of in-vivo operations.
The author declares no conflict of interest.

©2016 Ventura, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.