MOJ
eISSN: 2374-6912


Research Article Volume 3 Issue 5
1Bioplast Medical, LLC, Chesterfield, USA
2Wound Care Center, Grant Hospital, USA
3Department of Cancer Epidemiology, Moffitt Cancer Center, USA
Correspondence: John J Wille, Bioderm Technologies, Inc, 9 Chesterfield Georgetown Rd, Chesterfield, NJ 08515, USA, Tel 6092611488
Received: August 15, 2016 | Published: December 20, 2016
Citation: Wille JJ, Burdge JJ, Park JY. Wound healing of chronic leg ulcers is stimulated by wound edge continuity with adult cultured epidermal autografts. MOJ Cell Sci Rep. 2016;3(5):127-133. DOI: 10.15406/mojcsr.2016.03.00069
Epidermal stem cells isolated from adult human skin present an opportunity to heal a variety of chronic wounds. Significant progress has been made in the serum-free culture of adult keratinocytes and their use to produce living three-dimensional cultured epidermal autologous skin grafts for treatment chronic leg ulcers. We present new retrospective studies showing that rapid healing of chronic venous stasis leg ulcers is stimulated by close association of living cultured autograft implant edges with host ulcer wound edges.
Keywords:adult keratinocytes, cell banking, chronic wounds
The sine qua non for any commercially viable regenerative medicine application is a sustainable source of adult stem cells. An epithelial stem cell is defined as the cell of origin for terminally differentiated cells in the adult tissue.1 We believe this definition has been met by our methods of isolation and cloning of adult epidermal keratinocytes, which exhibit a multi-generational proliferative life span and undergo parasynchronous terminal squamous differentiation under defined serum-free culture conditions.2 At present, the best source of safe and reliable epithelial stem cells are autologous adult epidermal keratinocytes re-engineered as a biological dressing, a cultured epidermal autograft (CEA), useable for treatment of chronic wounds. The term biological dressings refers to the field of biomedical research and practice that focuses on the use of cell culture-derived tissue constructs to replace, restore and regenerate tissue loss due to damaged or diseased tissue cells. Biological dressings have had an important impact on outcome and efficacy of wound healing, and on the design and choice of the best clinical pathways for treatment of burns and other hard-to-heal wounds.3,4 To date, tissue engineered bilayered skin composite skin grafts using allogeneic keratinocytes have show only marginal wound healing benefit relative to standard of care treatment.5–11 Use of allogeneic epidermal cells requires pooling of neonatal keratinocytes from many different human sources from cell bank stocks. This carries many serious safety and health concerns including immunological rejection, and the possibility of disease transmission from unscreened pathogenic bacteria and viruses. To prevent such consequences, costly screening and quality control tracking systems are required reducing the commercial potential of such products. Moreover, allogeneic epidermal keratinocytes transplanted to host wounds are only of transient benefit and are not permanently integrated in to the hosts healed tissue.12 Recently, an innovative technique of cell therapy in which allogeneic keratinocytes and fibroblasts are sprayed on to debrided chronic leg ulcers wounds also showed only slight improvement in the rate or time to complete wound closure over and above compression therapy, the standard of care,13 and in a 24-week follow up, wound closure occurred for 43% of patients receiving cells versus 35% for those receiving vehicle.14 Previously, we reported15 using adult epidermal cells obtained from normal adult skin to treat chronic venous leg ulcers. The techniques and methods used to construct an autologous biological dressing composed entirely of autologous epidermal keratinocytes has been recently described.16 The methodology differs substantially from both previous biological dressings composed of allogeneic epidermal keratinocyte and also from biological dressings composed of autologous epidermal keratinocytes,17–21 including the use of serum- free culture medium, absence of an organotypic matrix or xenobiotic such as cholera toxins, and irradiated mouse feeder layer cells. These improvements were employed before2 to construct a serum-free adult CEA to heal chronic venous stasis leg ulcer. Here, we have evaluated our previous clinical data seeking correlations among a number of variables, including:
The results of these analyses indicate that accelerated wound closure is sponsored by contact of the implanted living three-dimensional epidermal sheets at the perimeter with the host debrided wound edges. We interpret this finding as suggesting that graft-induced wound healing occurs through stimulation of the hosts wound edge cells and eventual integrating of graft epidermal tissue with the surrounding “activated” host keratinocytes. These results are supported by our analysis of four independent cases requiring secondary grafting from patient’s cryopreserved and banked cells.
Methods related to the clinical trial results were previously reported and are briefly summarized here.2
Serum-free culture media MCDB 153 and novel HECK 109 and Hepes-buffers were individually prepared in the laboratory according to published recipes. Recombinant human growth factors (epidermal growth factor, EGF, insulin-like growth factor, IGF-1 and beta-TGF) and Hepes buffer were all purchased from Sigma –Aldrich, St. Louis, Mo). Dispase (Collagenase Type IV) was purchased from (Sigma-Aldrich, St. Louis, MO).
Patient recruitment and evaluation
The clinical protocol was reviewed and approved by the Institutional Review Board of Grant Hospital WCC (Wound Care Clinic), Columbus, OH. Given the small size of the study and known healing complications, patients with a BMI greater than 38 were excluded. All subjects with a history of venous stasis ulcers and who had failed repeated standard of care, i.e., compression therapy treatment for greater than three month’s duration were screened at the Grant Hospital WCC. These subjects had clinically confirmed venous insufficiency (venous duplex evaluation with reflux) without diminution of arterial flow. All of the ulcers were of the lower extremities and measured at least 2.5cm in one axis with a depth of up to 4-6mm but without exposed fascia, bone or tendon. None of the subjects had ongoing systemic or local infections as confirmed by quantitative cultures, or any history of recent treatment with immune-suppressants or steroids. All prospective participants had a standard set of physical evaluations and blood chemistries (SMAC10). All subjects exhibited normal blood chemistry results and all subjects had a body mass index of <36 and >16. A total of 22 patients were initially randomized. Seven patients failed to return for follow-up treatment after the first week. The remaining 15 patient were subdivided with 10 patients were randomized to the CEA application group (AG), and five were randomized to the control group (SCG). All 15 remaining subjects (the prospective target subject number, 10=AG; 5=SCG) completed the study for the 12 evaluation weeks.
Photograph and tracings
Tracings and photographs of the unclosed wounds were made by application of a clear plastic acetate sheet over the wound and estimates of wound depth were made by use of a calibrated probe. The data generated from these methods were stored and analyzed into a computer program (IMSI TurboCAD V). Wound outlines were extracted topographically by the program, and surface area (mm2) and volume (mm3) computed. Wound volume was measured using x/y/z tomography using the measured depth and assumed that the wound sides were 45" angles.
CEA production
A normal skin biopsy was used for CEA production. It consisted of a 1.5cm2 tissue sample taken from the iliac crest or back of the upper arm. Biopsies were shipped by express mail to the production facility (Mayo Clinic, Rochester, MN). The CEA was prepared by modification of a previously described method.2 The resulting in vitro produced completely reformed epidermis is released enzymatically (Dispase) from the flask, transferred, basal cell side up, to sterile petrolatum gauze backing, and immediately shipped from the Mayo Clinic, Rochester, MN via express mail to the Grant Hospital WCC, Columbus, OH. Upon arrival the grafts were applied to the AG patients’ debrided wounds.
Cryopreservation and cell banking
It was necessary to establish and maintain a cell bank of patients frozen cells. Thus, any cells that do not need to be cultured for grafts may be frozen in liquid nitrogen. A cell suspension at 2 to 4x106/ml in complete HECK 109 are diluted with an equal volume of freezing medium (FM) composed of: 60% complete HECK 109 medium, 20% Dialyzed fetal bovine serum (dFBC), and 20% DMSO (dimethylsulfoxide). The final concentrations are then: cells (1 to 2x106/ml) in 80% complete HECK 109 medium, 10% dFBS, and 10% DMSO. Thaw cells rapidly by hand twirling in a 37°C water bath. Transfer the cell suspension to a 15ml conical centrifuge tube with approximately 10ml of cold CCS solution. Centrifuge for 5minutes at 1000rpm in a cold centrifuge, Aspirate the supernatant and bring the cell pellet up in 10ml of complete HECK 109 medium and plate in to fibronectin (2-20ug/ml) coated flasks.
Ulcer treatments- standard and CEA applications
Prior to grafting, both the AG and the SCG patients received debridement of the peripheral and central wound-area. Following this debridement, the CEA was applied to the AG patients’ wounds with the basal cell layers in direct contact with the wound bed. Four-layer foot to knee compression wrap (Profore®, Smith and Nephew Ltd.) was applied with a saline dressing overlaying the wound directly, with or without the CEA. This standard of care with pre-treatment reduction/elimination of resident pathogens has been shown by WCC protocols to generally be effective in wound management/closure.
Linear regression analysis of the clinical data was performed as either one-variable or two variable programs using a Texas Instrument (TI-30X IIS) to obtain r-values.
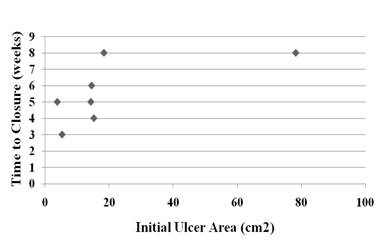
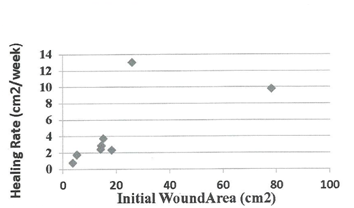
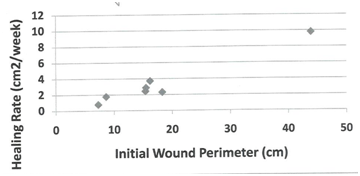
Demographic parameters and their correlation to rate of healing at time of closure. Table 1 below present’s demographic data for 10 subjects randomly assigned to the autograft group (AG), who received the cultured epidermal skin autograft (CEA) along with a column showing the calculated healed rate (HR) at the end of the 12 week study period. The healed subject data were further employed to test for possible statistical correlations by linear regression analysis (two-variables) between patient’s age, gender, wound age at time of grafting, and IWA (cm2) of healed wound, and healing rate (cm2/week) at the time of wound closure (weeks). No significant differences were found in the rate of wound healing between male (r = 2.1 ± 0.8) and female subjects (r = 5.1 ± 3.4), patient age (r2 = 0.32), although the oldest patient (subject 012) had the slowest HR (0.75). Age of patient’s wound at time of grafting (r2 = 0.18) was not significant as tested by linear regression analysis. As expected, there was a highly significant linear correlation between IWA and IWV as tested by linear regression analysis (r2 = 0.99). Table 2 present tabular data showing no significant correlation between wound age at time of treatment for healed wounds in the AG grafted patients and their corresponding healing rate (r2 = 0.18). Figure 1 presents a scatter plot for the eight healed subjects in the AG group plotted against the time of wound closure. There is a high degree of scatter among the data point, and linear regression statistical analysis indicated there was no significant linear correlation (r2=0.31). Also, linear regression analysis also indicated no significant correlation of IWV of host debrided wounds and time of wound closure of autografed wounds (data not shown). Figure 2 present data tested statistically by linear regression analysis showing a linear correlation exists between patient IWA and rate of healing at time of wound closure for AG. There is a weak positive correlation between IWA and healing rate of wound that closed during the 12-week study period (r2= 0.45). This appears to be due to one outlying data point (subject 06). This individual had a complete wound closure within two to three weeks of grafting. This was for a sizeable wound area (26 cm2). We have excluded subject 06 on the grounds that the average age of the healed subjects was 65.7 ±15.3 years, whereas this subject was at 28 years old, the youngest male patient grafted that healed. With the removal of this outlier a significant linear correlation was found for the remaining 7 subjects (r2 = 0.96). This result led us to seek further confirmation of a positive correlation of wound perimeter and healing rate. For this purpose, we used wound tracings obtained from photographs of each subject’s wounds to calculate perimeter. Figure 3 presents these results showing a positive linear correlation between initial host wound perimeter and healing rate (r2 = 0.96). Again, we have excluded the outlier data point for Subject 06 as discussed above. The results appear to suggest that grafting of an autologous cultured epidermis directly stimulates the host’s debrided wound edge as the epidermal keratinocytes of each come in direct and intimate contact with each other. Table 3 presents demographic data for 5 subjects randomly assigned to the standard of care group (SCG), and who received only high compression bandaging along with a column with their corresponding calculated healing rate (HR). All other patients failed to heal during the study period except one subject (20%, 1/5, Subject 16), who healed at week 12 of the 12 -week study. The demographic data were employed to test for possible statistical correlations between patient’s age (PA), gender (G), and wound age (WA) at time of grafting and the calculated healing rate, HR (cm2/week). There was no significant difference between patient age and rate of healing (r2 = 0.05), or between males (N=2, Xav= 0.67 ±0.15) and female (N = 3, Yav= 0.79±0.29) in rate of wound healing nor with age of patient’s wound at time of standard of care and healing rate at end of 12-week trial (r2 = 0.28). There was a weak positive linear correlation between IWA and IWV (r2 = 0.7) as expected. Since only one patient (Subject 016) healed completely during the study period, no correlations can be sought between time of closure and healing rate. It should be also noted that the only ulcer wound that closed was the smallest in size and healed at the slowest rate under the standard of care treatment regime (high compression bandaging). There was no significant linear correlation between IWA and healing rate for the five subjects of SCG based on the calculated rate at the end of the 12-week study period (r2 = 0.41).
Patient ID |
RG1 |
PA2 (yrs) |
G3 |
WA4 (Year/Month) |
IWA5 (cm2) |
IWV6 (cm3) |
IWP7 (cm) |
HR8 (cm2/wk) |
2 |
AG |
69 |
F |
2005/Jan |
15.16 |
4.4 |
16.2 |
3.7 |
4 |
AG |
54 |
M |
2000/May |
18.32 |
5.86 |
18.3 |
2.29 |
6 |
AG |
28 |
M |
2003/Jun |
26 |
7.15 |
23.5 |
13 |
8 |
AG |
57 |
M |
2000/May |
110.19 |
63.36 |
NH |
|
11 |
AG |
78 |
M |
2002/Jan |
14.45 |
3.61 |
15.5 |
2.84 |
12 |
AG |
83 |
M |
2002/Feb |
3.74 |
1.03 |
7.29 |
0.75 |
17 |
AG |
58 |
M |
2000/Jun |
14.22 |
2.13 |
15.4 |
2.41 |
18 |
AG |
81 |
F |
2000/Apr |
78.22 |
41.07 |
43.9 |
9.78 |
21 |
AG |
34 |
M |
2003/Mar |
19.18 |
5.75 |
NH |
|
22 |
AG |
77 |
F |
2000/Mar |
5.23 |
1.57 |
8.6 |
1.74 |
Table 1 Demographic data for subjects for the auto grafted group (AG) and their corresponding healing rates20
1RG: Randomization Group; 2PA: Patient Age; 3G: Gender; 4WA: Wound Age; 5IWA, Initial Wound Area 6IWV, Initial Wound Volume; 7IWP, Wound Perimeter; 8HR, Healing Rate For Closed Wounds; 9NH-Not Healed at the Termination Of The Study.
Subjects (AG) |
Wound Age (months) |
Healing Rate (cm2/week) |
2 |
17 |
3.7 |
4 |
60 |
2.29 |
6 |
75 |
13 |
8 |
5 |
0 |
11 |
14 |
2.84 |
12 |
26 |
0.75 |
17 |
6 |
2.41 |
18 |
4 |
9.78 |
21 |
39 |
0 |
22 |
3 |
1.74 |
Table 2 Healing rates and wound age of healed wounds for subjects in the autografted group (AG)
Analysis of patients receiving secondary CEA graft
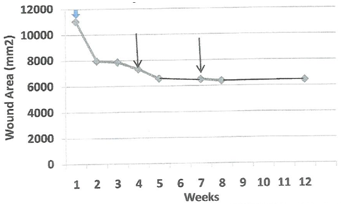
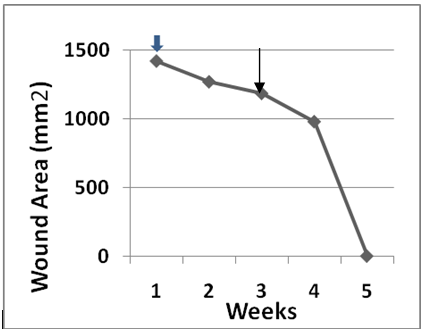
An important outcome of our earlier clinical trial was the discovery that the patient’s own basal epidermal keratinocytes could be cryopreserved, later thawed out and processed to make a useable CEA grafts. These secondary grafts were only employed to treat ulcers that failed to progress toward wound closure. Four patients22,23 required a secondary graft. Figure 4 plots wound area for patient 08 as a function of weeks after a primary CEA graft was initiated (blue arrow). The progress of wound closure was examined weekly then clinical director decided that it was too slow at week 4 and again at week 7 (see arrows). The rate of wound closure between the primary and secondary and between the secondary and tertiary graft derived from the calculated slopes, m1 and m2 were13.3cm2/day and 3.8cm2/day, respectively. Although the grafts were viable, no further increase in the rate of wound closure occurred after the third graft with less than 50% wound area closure. Figure 5 plots wound area for patient 017 as a function of weeks after a primary CEA graft was initiated (blue arrow). Progress of wound closure was examined weekly then ? it was decided that it was too slow at week 4 (see arrow). The rates of wound closure between the primary and secondary and after the secondary CEA graft shown by the calculated slopes, m1 and m2 were 1.6 cm2/day and 14 cm2/day, respectively. In this instance, application of the secondary graft accelerated wound closure. The wound closed by week 5 of the study. Figure 6 plots wound area for patient 018 as a function of weeks after a primary CEA graft was initiated (blue arrow). Progress of wound closure was examined weekly when again it was decided that it was too slow at week 5 (see arrow). The rates of wound closure between the primary and secondary and after the secondary CEA graft as shown by the calculated slopes, m1 and m2 were 19.8cm2/day and 4.2cm2/day, respectively. Like patient 08 application of a secondary graft exhibited a slower rate of wound closure but did result in wound closure at week 8 of the study. The healing rate of 4.2cm2/day is actually four times greater than the rate observed for wounds treated by compression therapy alone in this study(Table 3). Figure 7 plots wound area for patient 021 as a function of weeks after a primary CEA graft was initiated (white/blue arrow). Progress of wound closure was examined weekly when it was decided that it was too slow at week 3 (see arrow). The rates of wound closure between the primary and secondary and after the secondary CEA graft shown by the calculated slopes, m1 and m2 were 7.4cm2/day and 2.0cm2/day, respectively. The wound finally reached greater than 90% closure by week 12 of the study. Again note, the rate of 2.0cm2/day is actually twice the rates observed for wounds treated by compression therapy alone in this study (see Table 3). Table 4 presents a summary of the factors affecting the rates of healing of secondary auto graft data. The data were arranged by rank order of the largest to smallest initial wound area in relation to the rate of wound area closure for the primary and secondary CEA rates and their respective percent closure at week 12 of the study. Linear regression analysis indicates that there is a significant linear correlation (r = 0.77) between initial wound area and primary graft rate of wound closure. For secondary grafts the high rates observed for the primary grafts with the three largest initial wound areas were 3-4 fold lower in the secondary graft rates. By contrast, the one case where the primary graft had the smallest initial wound also had the smallest primary graft rate of wound closure followed by the largest rate of wound closure for the secondary graft.
Patient ID Number |
RG1 |
PA2 (Yrs) |
G3 |
WA4 (Year/Month) |
IWA5(cm 2) |
IWV6 (cm3) |
HR7 (cm2/wk) |
3 |
SCG |
80 |
F |
2000/Jun |
17.35 |
3.82 |
1.18 |
7 |
SCG |
44 |
M |
2010/May |
8.96 |
2.92 |
0.52 |
14 |
SCG |
42 |
F |
2000/Sep |
20.96 |
8.91 |
0.5 |
16 |
SCG |
83 |
F |
2000/Mar |
1.98 |
0.5 |
0.177 |
19 |
SCG |
73 |
M |
2000/May |
14.89 |
8.21 |
0.82 |
Table 3 Demographic data for subject in the standard of care group (SCG) and their healing rates and the termination of the 12-week study
1RG: Randomization Group; 2PA: Patient’s Age; 3G: Gender, 4WA: Wound Age, 5IWA: Initial Wound Area; 6IWV: Initial Wound Volume; 7HR: Healing Rate at the End of the 12-Week Study; 8Single Case of Closed Wound at 12-Weeks.
Patient ID |
Initial Ulcer Area (Mm2) |
Initial Graft Slope (Cm2/Day) |
Second Graft Slope (Cm2/Day) |
Percent Closure at Week (N) |
8 |
11,019 |
13.3 |
3.8 |
41.7 (12) |
18 |
7,822 |
19.8 |
4.2 |
100 (8) |
21 |
1,918 |
7.4 |
2 |
92.2 (12) |
17 |
1,422 |
1.6 |
14 |
100 (5) |
Table 4 Parameters affecting the rate of wound closure of secondary auto grafts
This retrospective report extends the results of our recently published clinical trial2 by analyzing the calculated healing rate achieved during the 12-week trial period for the eight closed CEA-treated wounds and for the five subjects of standard of care group. Statistical analyses were then performed to determine if correlations existed between patient’s own demographic data and the healing rate of their autographed wounds. Linear regression analyses revealed that there was a strong positive correlation between the initial wound area and initial wound volume, which were independently assessed. This outcome was expected as the depth of venous stasis ulcers tend to be relatively shallow with little dermal invasiveness making the volume calculation a simple function of the depth measurement and a geometric consequence of the ratio of square of the radius of the surface area to the cube of the radius of the volume. It also suggests that deviation in wound shape was only minimal. The gender of the subjects was not positively correlated to wound healing either for the eight graphed subjects or the five control subjects. A possible gender correlation might have been anticipated if endocrine factors played a significant role in speed of wound closure. No significant correlation was found between subject’s age and wound healing rate of the grafted group or pooled age for both the SCG and AG data (r2=0.3, N=13). In fact, the only wound healed in the SCG was the oldest subject aged 83years old with the smallest wound and the slowest rate of healing. Another correlation was sought between subject’s duration of wound and its corresponding healing rate. Again, no significant linear correlation was found for either the autografted group or the standard of care group with a wound age ranging from 3 to 72months. The inclusion criterion restricted wound age to greater than 3months duration. Past wound history appears to be a non-factor in response to high compression bandaging (HCB) alone for the SCG subjects or for subjects that had both combined HCB combined treatment with a cultured epidermal autograft (CEA). Importantly, no significant linear correlation was found between a patient’s initial wound area (IWA) and the time (weeks) elapsed till wound closure for those ulcers treated with a cultured epidermal autograft. This uncoupling of IWA to time of completion of wound closure suggests that something other than mere wound size is at play when a debrided wound is treated with a living autologous epidermal graft. As only one control HCB subject ulcer healed, no correlation could be statistically examined. By contrast, a strong positive linear correlation was found between initial ulcer wound size (area) and healing rate for seven (7) subjects with CEA-treated wounds. We excluded one outlier (Subject 06) whose wound closed in an amazing 2-3weeks after graft implant. Our results demonstrate that the wounds having a larger initial wound area have faster healing rate. One possibility is that the contact area between the CEA graft implant and surrounding host tissue plays an important role in accelerating wound closure. We further tested this idea by measuring initial wound perimeter. A significant linear correlation was found between initial wound perimeter and healing rate for the 7 subjects whose wounds closed in the 12-week study period. A non-significant linear correlation was found (r2=0.20) for all five SCC subjects between IWA and healing rate, If the largest wound (Subject 014) is dropped as an outlier, a highly significant linear correlation (r2=0.96) was found. Although the size of the SCG sample is small, it is likely that compression therapy also reduces the distance between host tissue wound edges for wound areas less than 20cm2 expediting wound closure. As reported earlier,20 in a one year follow-up one more autografted patient (Subject 08) healed at 30weeks. Coincidently, this rate of closure is close to the 29weeks predicted from the initial healing rate (3.83cm2/week). Recall that this wound required a total of three CEA grafts. Moreover, one more patient that received HCB therapy (Subject 014) healed at 32weeks, which is close to the 42weeks predicted from the initial healing rate (0.50cm2/week). Earlier, Panuncialman et al.23 reported a wound healing rate of 0.99±1.18mm per week for wound edge biopsied chronic leg ulcer or 0.1cm/week and an estimated 0.32cm2 of wound area per week. This compares favourably with a mean of 0.6±0.4cm2/week for the five patients that received HCB therapy. By contrast, the mean healing rate for the eight subjects of CEA group with closed wounds was 4.6±4.1cm2 per week. The engrafting of a living cultured epidermal autograft enhanced the rate of wound healing by approximately one order of magnitude, i.e.7.7times. Chronic VSU treated with a composite skin substitute (Epigraph®), also exhibited a positive correlation between initial healing rates and predicted time of wound closure, using ellipsis correction yielded a value of 0.1cm/week, or an estimated 0.32cm2/week for wound area.7 We propose that engraftment of either a bilayered? or autologous CEA places the living epithelial keratinocytes into close proximity of the host’s epithelial tissue thereby stimulating migration of the activated host basal layer to repair and close the gap. This explanation may also account for the results of our analysis of four independent cases requiring secondary grafting of cryopreserved and banked cells. The exact mechanism by which close approximation of patient’s own keratinocytes with the debrided wound edge accelerates wound healing is not known.
None.
The author declares no conflict of interest.

©2016 Wille, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.