MOJ
eISSN: 2374-6912


Mini Review Volume 3 Issue 5
1Department of Medical Biochemistry and Molecular Biology, Suez Canal University, Egypt
2Faculty of Science, Suez Canal University, Egypt
3Faculty of Pharmacy, Suez Canal University, Egypt
4Department of Human anatomy and embryology, Suez Canal University, Egypt
Correspondence: Nora Hosny Ahmed, Assistant lecturer of Medical Biochemistry, Suez Canal University, Ismailia, Egypt, Tel 1006906656
Received: August 17, 2016 | Published: October 17, 2016
Citation: Ahmed NH, Shams OS, Elbaz AR, et al. Brown adipose tissue- what is known and what to be known? MOJ Cell Sci Rep. 2016;3(5):122-125. DOI: 10.15406/mojcsr.2016.03.00068
Adult human brown adipose tissue has been known for a long time as a vestigial organ with limited or no function that found with opulence in newborn and infants, helping them controlling their body thermogenesis without shivering, followed by its gradual disappearance with age (disparate form animals like rodents which keep BAT in adult life). Recently BAT existence, distribution and activity has been unraveled accidentally. There by researches on BAT demonstrated undeniable links to human metabolism and body composition. The fixed facts regarding body metabolism and energy homeostasis that have been granted for centuries are about to exhibit different perspectives. BAT discovery ignited a new line of research focusing on modulating energy expenditure and hence controlling many metabolic phenomena. Here we offer a brief review of what have been reported regarding BAT and it activity with pointing to novel challenges that need to be unveiled.
Adipose tissue is a loose connective tissue classified to white adipose tissue (WAT), which is an active endocrine organ acting as an energy storage depot (Figure 1), with a small amounts of Brown adipose tissue (BAT) (Figure 1).1 BAT is found in almost all mammals. In contrast to white adipocytes, which contain a vast single lipid droplet, brown adipocytes contain numerous smaller droplets, a much higher number of (iron-rich) mitochondria, which gives BAT its brown appearance,2–4 besides it expresses uncoupling protein 1 (UCP1) that is located in the inner mitochondrion membrane and uncouples the rates of substrate oxidation and adenosine triphosphate (ATP) production by favoring protons’ loss and thus energy liberation in the form of heat. Brown fat also contains greater number of capillaries than white fat, to supply the tissue with nutrients and oxygen together with spreading and distributing the produced heat throughout the body.2
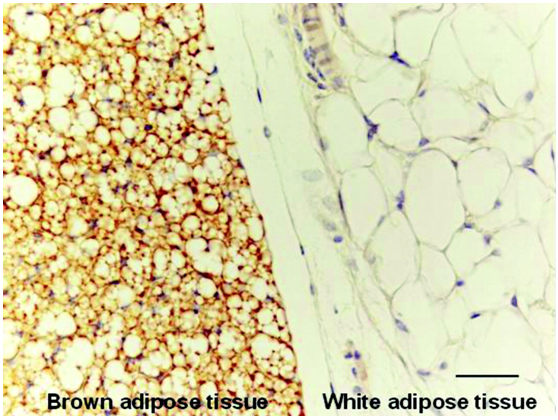
Figure 1 BAT has many lipid droplets compared to the vast single lipid-filled space of WAT (ahajournals.org(4))
Classification of brown adipose tissue refers to two distinct cell populations, defined by both cellular morphology and anatomical distribution, with similar functions. Both share in common the presence of small lipid droplets and copious amount of iron-containing mitochondria. The former have a common embryological origin with muscle cells, found in larger "classic" deposits. This ‘’classical" brown fat is situated in highly vascularized deposits in consistent anatomical locations, such as the neck, between the shoulder blades, supraclavicular area, surrounding the kidneys, and along the spinal cord.5 This is the smallest of the two types, and has enormous number of small lipid droplets. The second evolves from white adipocytes under the control of sympathetic nervous system stimulation. These adipocytes are localized interspersed in white adipose tissue and are also called 'brite' or 'beige' adipose tissue. Beige fat is the sympathetically inducible cell type that is dispersed throughout the existing adipose tissue. It varies over a great range of lipid droplets size, and has a greater abundance of lipid droplets compared to mitochondria giving it a lighter brown appearance.5
Brown adipose tissue is temperature regulator organ especially in hibernating mammals, being responsible for non-shivering thermogenesis (NST). However, in man it present in abundance in newborns, BAT was believed to disappear rapidly with age, with old believe of their nonfunctioning role in adult humans.6 In 1977, numerous studies on rodents led to the belief that an impaired maintenance of energy expenditure (i.e, lower uncoupling level and therefore heat production) was critical in regulating metabolic efficiency leading to obesity. For instance, the inability of young mice to maintain and control their body temperature on cold exposure is predictor of an obese phenotype later in life.7 Late in 1979, Rothwell and Stock performed a series of experiments examining rodents on high-fat, high-sucrose diet.8 They showed that in spite of consuming about 80% more energy, the high-fat, high-sucrose -fed rats had only a 27% more weight gain compared to chow-fed rats. Moreover, similar to animals with long term exposure to cold, high-fat, high-sucrose fed rats’ demonstrated increased sympathetic activity and metabolic rates correlated with an increase in the activity of BAT. This study unravel the initial clues suggesting a significant role of BAT in controlling body weight and composition in rats. High-fat, high-sucrose fed animals had an increment in BAT mass, elevated levels of skin and abdominal temperatures, and four-fold-more levels of norepinephrine- stimulated free fatty acid release. Besides, in the same article, Rothwell and Stock confirmed the existence of functional BAT in an adult human by demonstrating thermogenesis at the skin surface using infrared thermography after the ephedrine administration.8 Although these results were highly criticized at that time, they raised the question about the potential significance of BAT in humans and its role in the energy balance regulation.4
Late in 1990s, pharmaceutical companies evolved compounds addressing adipose tissue–selective β3-adrenoceptors as a claimed pharmacological tool used to increase energy expenditure through activation of BAT and thus losing body weight. In man, β3-adrenoceptors are situated predominantly on BAT with no or few receptors on WAT. β3-Agonists demonstrated a bit positive metabolic effects such as improving insulin action and increasing fat oxidation but it generally failed to increase 24-hour energy expenditure and hence BAT activity.9
In mid-2000s, studies in nuclear radiological medicine using labeled 18fluoro-2-deoxyglucose positron emission tomography/ computed tomography (18F-FDG PET/CT) scanning revealed the existence of BAT in adult humans. This FDG uptake pattern in the supraclavicular area fat, was firstly considered as an artifact by radiologists who were concerned with identifying active tumors. However, radiologists reported that the presence of BAT had relation to ambient outdoor temperature10 and that, unlikely to tumors, 18F-FDG uptake could have pharmacologically non-competitive blockage by β-blockers in rats and humans.11,12 By 2009, several research groups studied the existence and activity of BAT in adult humans using 18F-FDG PET/CT.13-17 All these groups demonstrated actual metabolically active BAT storage depots in the cervical-supraclavicular regions, and some proved the existence of true BAT by identifying the expression of UCP1 with BAT histological characteristics. For example, Virtanen et al.15 demonstrated that exposure to cold made increment in glucose uptake up to 15-fold in para-cervical and supraclavicular adipose tissue. Adipose tissue biopsies were collected from 3 out of 5 candidates, UCP1 expression by mRNA and protein levels immuno histochemistry confirmed that the tissue demonstrated in the PET/CT scans was true BAT.16 Besides, Cypress et al.13 analyzed numerous 18F-FDG PET/CT consecutive scans from large group of patients and demonstrated positive BAT scans in 7.5% of the participating women and in 3.1% of the participating men.13 Additionally, van MarkenLichtenbelt et al.14 studied group of men under thermoneutral conditions (22°C) compared to under mild cold exposure conditions (15°C for 2 hours) using 18F- FDG PET/CT. BAT activity was reported in most of the studied subjects during cold exposure but in no participants under normal temperature levels.14 Moreover, the same research group reported a positive association between basal metabolic rate and BAT activity at normal temperature levels or during cold exposure, meanwhile an inverse relationship comparing BAT amount and function to body mass index was reported.
Another evidence that BAT may have a role in the regulation of energy homeostasis and body weight in humans developed from analyzing patients with pheochromocytoma who demonstrates a clear biological model for overstimulation and reactivation of BAT (mass and activity) and body weight loss(18-21). In 1980s, Lean et al.17 supposed that the high circulating of noradrenaline, well known to subjects with pheochromocytoma, was associated with rejuvenation of BAT and contributed to the considerable weight loss accompanied with the pheochromocytoma even in lean subjects.18,19 More importantly, this group reported an increment in UCP1 expression in the BAT of these patients and in the basal mitochondrial oxygen uptake nearly similar to the fully uncoupled state (measured after adding the uncoupling agent carbonyl cyanide paratrifluoromethoxyphenylhydrazone). Moreover by 2008, a group of researchers provide a rebuttal on BAT’s role in mediating energy expenditure in patients with pheochromocytoma by noting that the demonstrated FDG active uptake in the supra-clavicular BAT, after the tumor resection, was no longer apparent in most of patients.22
In trial to identify BAT localization and distribution through using the fore mentioned 18FDG-PET scans and data collected from human autopsies, numerous BAT depots have been identified. In newborn and infants, BAT depots localized in, but are not limited to: supraclavicular, suprarenal, para-aortic, pericardial, trachea, kidney and around the pancreas.23 These depots progressively shifted to more white fat like those in adult human life. In adults, the depots that are most often found by FDG-PET scans are the supraclavicular, paravertebral, para-aortic mediastinal, and suprarenal depots.24,25 In contrast to animals like rodents, the humans ‘BAT’ depot may consist of both classical brown adipocytes and brown-like adipocytes dispersed through white adipose tissue.
A long way off, cold exposure is considered as the strongest stimulator of BAT in humans,26 but extensive research are directed on finding alternative activators of BAT. Cypess et al.13 reported that mirabegron, a β3-adrenergic receptor agonists and an approved drug for treating overactive bladder, induce higher BAT metabolic activity, higher resting metabolic rate (RMR) correlated with BAT thermogenesis and proposed that mirabegron may be a promising candidate in BAT induction.27
During cold exposure process, a remarkable quantities of glucose and lipids are consumed, far exceeding those known to be consumed by the capacity of liver and skeletal muscles. Bartelt et al.26 uncovered that BAT activity is the answerable for this highlighted gap, referring to its role in controlling plasma lipid profile and striving against obesity through inducing a specified metabolic program.28 Besides, Matsushita et al., 2014 observed that BAT activity had a significant influence on glucose homeostasis and metabolism in adult humans.29 In the mid of 2015, Schrauwen et al.24 suggested that BAT stimulation protects against hyperglycaemia and combats diabetes.26
By 2016, Lee et al.28 revealed how brown fat under effect of glucose utilization in adult humans is accompanied with increment of temperature level and heat production in a rhythmic circadian manner.30 Additionally, higher brown fat proportion correlates positively with lesser glycemic fluctuations, supposing that brown fat adipose tissue may act as buffering system for the rhythmic changes affecting daily glucose levels in circadian manner and helps in controlling body glucose balance over time. Besides, they revealed in the same study that glycated hemoglobin, as a marker of plasma glucose level over the past three-month, positively correlated with environmental temperature levels.30 Moreover, Takx et al.29 suggested that the increment in BAT activity is negatively correlated with arterial inflammation and atherosclerosis together with lower association with cardiovascular events.31
The ongoing studies have led to a dramatic shift in our believes regarding BAT in adult humans, this fuels a new line of research with interest in the potential of activating BAT to enhance energy homeostasis, therefore assisting in maintaining body weight and possibly combating a variety of metabolic diseases. Fostering the new era of brown fat biology, this instigate our concern to develop many questions: first is gender (noting the fact that progesterone is a potent uncoupling agent affect BAT biology, anatomical distribution, volume and activity? Then whether acute or chronic cold is the more robust stimulus for BAT activation? If sustained cold or alternative cold and hot intervals are more powerful regarding BAT activation? What about non-pharmacologic factors alter BAT activation (exercise, progesterone, capsaicin, ginger and caffeine)? Is there a mutual effect between BAT metabolism and hormonal metabolism (e.g. progesterone, insulin and thyroxin)? Does BAT status exhibit any change with pregnancy? How BAT activity and metabolism is specifically regulated? Will the exact time of postnatal activation influence the type, volume, distribution, and activity of BAT? What about oxygen status, angiogenesis factors effect and signaling pathways involvement in BAT status? Is there any other metabolic effects or functions to BAT? What about its cross relations with other body organs? Does BAT like WAT has any endocrine or secretory functions? What will be the health effect and hazards of BAT activation on the long run? What is the relation of different BAT genes polymorphism (UCP1, GLUT4) to the temperature profile, body mass index, lipid profile, diabetes mellitus and other metabolic problems? What is the link between BAT genomics, proteomics to obesity, glucose level fluctuations, diabetes, cardiac diseases, and metabolic syndrome? Is there a relation between BAT existence, activity and menopausal symptoms (hot flushes)? The next challenge will definitely be aimed at remodeling and activating BAT depots serving to modulate body energy, metabolism and alter or possibly prevent various metabolic diseases and health phenomena. Therefore the field of BAT biology still need extensive efforts to unravel whether BAT is a self-credible organ that can offer a hope for metabolic problems patients.
Sincere gratitude and appreciation goes to medical biochemistry department, FOMSCU with special thanks to the spirit of Professor Samir Abd El Moneim for sparking our enthusiasm to have further reading and studies in this field.
The author declares no conflict of interest.

©2016 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.