MOJ
eISSN: 2381-179X


Case Report Volume 4 Issue 6
1Health Information Technology Department, Republican Scientific and Practical Centre of Cardiology, Belarus
2Cytoplasmic Inheritance Department, Institute of Genetics and Cytology of the National Academy of Sciences of Belarus, Belarus
Correspondence: Tatiyana G Vaikhanskaya, Health Information Technology Department, Republican Scientific and Practical Centre of Cardiology, 220036, R Lyuksembourg str 110, Minsk, Belarus
Received: September 22, 2016 | Published: September 30, 2016
Citation: Vaikhanskaya TG, Sivitskaya LN, Danilenko NG, et al. Case report of malouf syndrome not associated with LMNA gene mutation. MOJ Clin Med Case Rep. 2016;4(6):144-147. DOI: 10.15406/mojcr.2016.04.00112
Malouf syndrome, currently known as dilated cardiomyopathy associated with hypergonadotropic hypogonadism (DCM-НН), is a rare congenital disorder with clinical signs including DCM phenotype, ovary dysgenesis in females or primary testicular failure in males, mental retardation, facial dysmorphism, skin lesions, bone abnormalities and occasionally marfanoid habitus. The disorder can be caused by mutation in the LMNA gene, encoding lamins A and C. In the present manuscript, we report the sporadic case of a young female with dilated cardiomyopathy, hypergonadotropic hypogonadism and primary amenorrhea, cognitive deficiency, body mass deficit, facial dysmorphism and subclinical hypothyroidism. Radiation exposure, mumps, diabetes mellitus, autoimmune diseases, and Turner’s syndrome have been ruled out. Specific features of mandibuloacral dysplasia, like bone dysplasia, are absent. The first-degree relatives are healthy. The clinical data suggested a laminopathy. Targeted next-generation sequencing was used to search for mutations in genes, associated with cardiomyopathy, including LMNA. However, genetic analysis did not confirm the assumption: mutation was not found. This data suggests that molecular mechanism of Malouf syndrome may be associated not only with LMNA mutations, but with other genes, probably functional partners of lamin A/C.
Keywords: lamin А/С gene, dilated cardiomyopathy, hypergonadotropic hypogonadism, cardiogenital syndrome, facial dysmorphism, malouf syndrome, next generation sequencing
LMNA, lamin A/C gene; DCM, dilated cardiomyopathy; HH, hypergonadotropic hypogonadism; NGS, next generation sequencing; MRI, magnetic resonance imaging; ECG, electro cardiogram; PVCS, premature ventricular contractions; LVEF, left ventricular ejection fraction; MAD, mandibuloacral dysplasia
Cardiogenital syndrome (Malouf syndrome) is a very rarely encountered syndrome which was first diagnosed in 1985 upon the examination of two sisters, with findings of hypergonadotropic hypogonadism, dilated cardiomyopathy, blepharoptosis and broad nasal base.1 Later, Narahara et al.,2 diagnosed another sporadic case with the same features. Malouf syndrome, also known as dilated cardiomyopathy-hypergonadotropic hypogonadism (DCMHH, OMIM 212112) syndrome, is a congenital disorder. The clinical features include congestive or dilated cardiomyopathy, ovarian dysgenesis in females or primary testicular failure in males, mental retardation, broad nasal base, blepharoptosis, skin lesions, skeletal abnormalities. The phenotype varies and not all features are present in every individual case. Occasional findings include a metabolic abnormalities, thyroid hemiagenesis, collagenoma, diabetes mellitus, and marfanoid habitus (tall stature with long and thin limbs, little subcutaneous fat, arachnodactylia, joint hyper extensibility, narrow face, small chin).1–8 Cardiogenital syndrome has two main features such as dilated cardiomyopathy and hypogonadism (hypergonadotropic pattern). The clinical symptoms of the disease are predominated by the following features: mental retardation (poor capacity to study and impaired language development in young age are followed by general decline in intellectual potential), abnormal ovary development (premature ovarian failure in females)accompanied by delayed puberty or primary testicular failure (in males), various osteocutaneous abnormalities (Marfan-like habitus) with unusually long and thin digits (arachnodactylia), facial dysmorphism (a narrow face and a small sized chin, retrognathia, blepharoptosis). Additional signs are chest disorder (as a narrow “keeled breast”, sloping shoulders) and adipose tissue deficit. Micrognathia and sloping shoulders may mimic atypical progeroid phenotype. However, patients with Malouf syndrome don’t suffer from severe growth failure, alopecia or rapidly progressive atherosclerosis. The prevalence of the syndrome is not fully defined, less than 1/1000000. About 20 families from around the world, whose members suffer from cardiogenital syndrome, have been described in literature. The cardiogenic Malouf syndrome can be caused by mutation in LMNA gene located on chromosome 1q21.2-21.3. It encodes the structural components of the nuclear lamina‒lamin A and C. Remarkably, mutations in LMNA have also been associated with at least 10 clinically distinct syndromes(termed as laminopathies), encompassing a wide variety of phenotypes, including several specific muscular dystrophy, DCM with conduction disorders, the dramatic “premature aging” phenotype of the Hutchinson-Gilford progeria syndrome, mandibuloacral dysplasia, and partial lipodystrophy. Quite frequently the mutations are connected with an overlapping these phenotypes. The LMNA mutation can manifest in various tissues such as cardiac and skeletal muscle, nervous, adipose and cutaneous tissue. LMNA-related DCM usually presents in early to mid-adulthood with symptomatic conduction system disease or arrhythmias. DCM patients with LMNA mutations have poor prognosis due to life-threatening ventricular tachyarrhythmias, progressive heart failure and high risk of sudden cardiac death. In cardiologist’s routine practice, a genetic diagnosis of LMNA mutation associated with DCM is a key aspect to assess the optimal treatment strategy and a timely preventive cardioverter-defibrillator implantation.
We describe a sporadic case of a young female with dilated cardiomyopathy, ovarian dysgenesis, mild mental retardation, body mass deficit, facial dysmorphism, and subclinical hypothyroidism. At the age of 24years, the patient was admitted to the clinic due to the first cardiac complaints (dyspnoea, fatigue, heart rhythm disturbance) and congestive heart failure. On physical examination she had blood pressure of 105/65 mmHg, heart rate (HR) 110 beat per min (BPM), a solitary crackles over the lung bases, and a systolic murmur at the apex. The height of the patient was 176cm with a weight43kg (body mass index - 13.9kg/m2). Symptoms of lipodystrophy were observed as uniformly proportional loss of subcutaneous fat. She also had a narrow chest and sloping shoulders, facial dysmorphism as a severe microretrognathia, a small chin, and a prominent beak nose. The patient had primary amenorrhea without the somatic stigmata of Turner’s syndrome. The secondary sexual characters of the patient were undeveloped. The medical history was negative for previous mumps, muscular dystrophy, diabetes mellitus, surgery or radiation to the pelvic region or autoimmune disorders. The female patient was the second child born from unrelated parents. The first-degree relatives of the patient (parents and elder sister) had experienced normal puberties and were healthy. There was no family history of menstrual disorders, intellectual deficit, dysmorphic features, congestive cardiomyopathy or sudden cardiac death.
At examination of the patient a 6-minute walk test was 415m. The 12-lead electrocardiogram (ECG) showed sinus tachycardia (heart rate 104 BPM) with left axis deviation, left bundle branch block (QRS duration 124ms), normal corrected QT interval and nonspecific ST-T wave abnormalities. Holter monitoring of ECG showed an intermittent left bundle branch block (to 33%/24h); frequent premature ventricular contractions (PVCs) to19600/24h, coupled PVCs (158 couplets/24h), ventricular triplets (44/24h), non-sustained ventricular tachycardia (4 to 7 ventricular complexes with HR max up to 130BPM). ECG-strip demonstrating ventricular arrhythmia is presented in Figure 1A. The patient underwent echocardiography which demonstrated left ventricular dilatation with global systolic dysfunction, hypokinesis and moderate mitral valvular regurgitation. Diastolic and systolic diameters of the left ventricle were 57 and 47mm, respectively. The left ventricular (LV) ejection fraction (EF) was 33% and LV global longitudinal strain was -11,6%. Cardiac magnetic resonance imaging (MRI) confirmed criteria of DCM: LV dilatation (LV end-diastolic volume 177 ml, LV end-systolic volume 124ml) and global systolic dysfunction (LVEF 30%). Hypertrabecular structure of myocardial apex was found also.MRI findings of cardiomegaly are presented in Figure 1B, Figure 1C. Endomyocardial biopsies were examined, and no genetic markers of 8 cardiotropic viruses using PCR method were detected. Histological findings showed non-specific diffuse changes in myocytes (their size and nuclei varied) and interstitial fibrosis, no conclusive evidence of inflammation. Hormonal evaluation included follicle-stimulating hormone (140.2IU/L; menopausal range 25.8-134.8IU/L), luteinizing hormone (70.5IU/L; menopausal range 7.7-58.5IU/L), estradiol (201pmol/L; menopausal range 0.1-505pmol/L). Thyroid function test was consistent with subclinical hypothyroidism (thyroid stimulating hormone 6.98mIU/L; normal range 0.35-4.94mIU/L). Mini-Mental State Examination cognitive test was 20 scores (11-19 scores: medium dementia; 20-23 scores: mild dementia). Ultrasonography revealed a moderate thyroid hypoplasia. The patient refused to receive hormone replacement therapy. Pelvic ultrasonography revealed hypoplastic uterus, and the bilateral ovaries could not be clearly seen. However, the patient was capable to menstruate only with the support of estradiol and progesterone. X-rays examination of collarbones, extremity phalanges and cranium was performed to exclude mandibuloacral dysplasia taking into account signs of severe microretrognathia and adipose tissue thinning (lipodystrophy). Mandibuloacral dysplasia (MAD; OMIM 248370) is a rare, genetically and phenotypically heterogeneous, autosomal recessive disorder characterized by skeletal abnormalities including hypoplasia of the mandible and clavicles, acroosteolysis, cutaneous atrophy and lipodystrophy. X-rays revealed no evidence of MAD-specific bone dysplasia, i.e. distension of fontanel cranial sutures, dental abnormalities, clavicle hypoplasia and distal phalange acroosteolysis findings are presented in Figure 2.
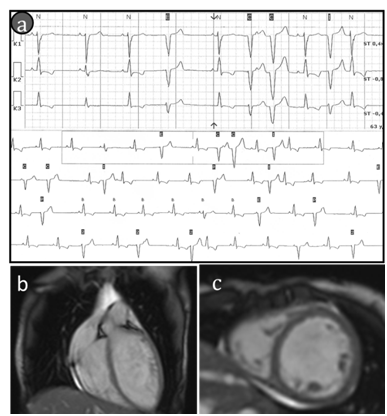
Figure 1Findings of cardiac examination.
(A)ECG strip demonstrates the premature ventricular contractions (to 100PVCs/1h). (B &C) cardiac MRIetc-cardiac MRI features of cardiomegaly (b-long-axis and c-short-axis post contrast views ,there is no sub-epicardial or mild-myocardial pattern of late gadolinium enhancement).
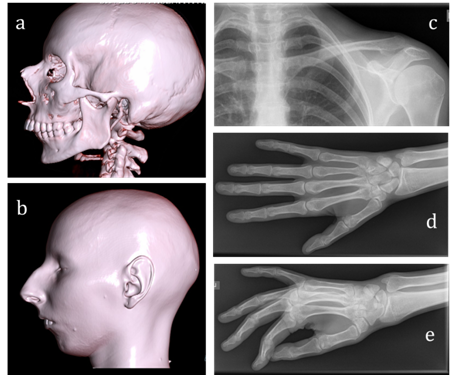
Figure 2Findings of X-ray examination.
A, B) Cranium tomography (normal cranial suture, micrognatia) with 3-D scans reconstruction. There is no any evidence of MAD-specific bone dysplasia C) Normal clavicle (D&E) Absent of distal phalange acroosteolysis.
Thus, the patient was presented with primary amenorrhea, hypergonadotropic hypogonadism, DCM, hypothyroidism, mental retardation, undeveloped secondary sexual characters, marked body mass deficit due to a significantly reduced adipose tissue; she also had a narrow chest and sloping shoulders, facial dysmorphism (micro retrognathia, prominent beak nose). These clinical data suggested a laminopathy.
Informed consent was obtained from patient for genetic investigation. Genomic DNA was used for next-generation sequencing on MiSeq System (Illumina Inc., San Diego, CA, USA). We used a custom-made panel of 46 genes associated with cardiomyopathies, including LMNA, the TruSight cardiomyopathy sequencing panel (Illumina Inc, San Diego, CA, USA). Coordinates of sequence data were on NCBI build37 (UCSC hg19). We have identified 110 variants, 24 of which were missenses and 1 located in splice site. No changes in LMNA gene were found in our patient. The Minor Allele Frequency (MAF) for all identified missense and splicing variants were greater than 1% in population database. The clinical role of these variants is not confirmed. After 3 and 6-month follow-up with guideline directed medical therapy the patient showed no progression of heart failure (NYHA II) and haemodynamic improvement (LVEF 45%, not clinically significant ventricular ectopy 180 PVCs/24h) therefore the planned cardiac resynchronization defibrillator therapy was delayed.
The clinical case has a manifestation typical for cardiogenital syndrome. This rare pathology relates to laminopathies, associated with LMNA mutations and numbering more than a dozen of here ditary disorders. Mutations in the LMNA gene cause a variety of disorders including dilated cardiomyopathy, muscular dystrophy, familial lipodystrophy, progeria, atypical progeroid syndromes, and mandibuloacral dysplasia. Phenotypic features in the present case are very similar to 3 cases reported by McPherson et al.,3 Nguyen et al.,4 & Chen et al.5 The heterozygous missense mutations (L59R, A57P) in the LMNA gene were identified by authors. Features common to these patients included premature ovarian failure, dilated cardiomyopathy, lipodystrophy, and progressive facial and skeletal changes involving micrognathia and sloping shoulders, but not acroosteolysis. The patients had some sings of progeroid syndrome. But severe growth failure, alopecia and rapidly progressive atherosclerosis were not observed. McPherson3 and her colleagues suggested that the phenotype represents a distinct laminopathy involving DCM and hypergonadotropic hypogonadism.
Genetic analysis has not detected LMNA mutation in our patient. All exons and their flanking regions as well as 5’-and 3’-untranslated regions were covered by the panel. Of interest, that Gersak et al.,6 reported sporadic case of DCM and hypogonadism in family from Slovenia. They described female patient with typical clinical features for Malouf syndrome: small chin, bilateral blepharoptosis, long digits and hypothyroidism. Nevertheless, no pathogenic mutations in LMNA gene have been found as well. Together with our patient it is the second reported case of sporadic DCM-HH associated with thyroid dysfunction; familial DCM-HH with thyroid hemiagenesis was first reported by Gursoy et al.7 It can be assumed that these patients form a new genotypic cluster distinct from laminopathies but nevertheless phenotypically overlapping with Malouf syndrome. The molecular etiology remains unknown in other indicated cases.1,2,7,8 Detection of such clinical cases indicates other genetic involvement in the pathogenesis of the Malouf syndrome. The term laminopathies defines a group of diseases associated with mutations in the genes coding for lamins, proteins associated with their post-translational processing, or proteins interacting with the lamins. Lamins and their partners are essential for chromatin organization, DNA replication, epigenetics, transcription, cell cycle regulation, cell development and differentiation, nuclear migration, and apoptosis.9 There are many functional and structural protein-partners of lamin A/C,10 some of them are included in the gene network (Figure 3). So molecular changes caused by mutation in partners will launch a major pathogenetic mechanism manifested in similar phenotype. We analyzed our patient for 46 genes associated with DCM. The sequencing panel consisted of sarcomere protein genes, nuclear lamin, ion channel, desmosomal and other genes. The lamin partner genes were not included. The tangled web of mutual protein-protein relations is an obstacle hindering explanation of the mechanisms underlying the development of laminopathies and other orphan diseases. Comprehension of all molecular connections is still a great challenge.
In conclusion, presented case report of Malouf syndrome is not associated with mutation in the LMNA gene. But the changes in the structural and functional partners of lamins are not excluded. To this day, the cardiogenital syndrome attributed to laminopathies according to the results of LMNA gene analysis. But some cases without LMNA mutations have already been described. This indicates the existence of cardiogenital syndrome with another genetic background. The low prevalence of Malouf syndrome, the lack of genetic researches in past reports, and the heterogeneity of clinical manifestations complicate the definition of the molecular mechanisms of pathology. We note that thyroid dysfunction already described for the cardiogenital syndrome not associated with LMNA mutations,6 may play the aetiological role (mental retardation?). We call on clinicians to focus special attention to this feature.
None.
The author declares no conflict of interest.

©2016 Vaikhanskaya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.