MOJ
eISSN: 2471-139X


Research Article Volume 10 Issue 1
1Department of General Surgery, Hospital Ángeles Lomas, México
2General physician, Hospital Ángeles Lomas, México
Correspondence: Alejandro Weber Sánchez MD, Department of General Surgery, Hospital Ángeles Lomas, Vialidad de la Barranca s/n C410, Valle de las Palmas, Huixquilucan, 52763, Estado de México, México, Tel +525543504996
Received: January 01, 2023 | Published: January 17, 2023
Citation: Weber-Sánchez A, Weber-Alvarez P. Modification of dermatome mapping method for the assessment of Inguinodynia treatment. MOJ Anat Physio. 2023;10(1):4-7 DOI: 10.15406/mojap.2023.10.00328
Introduction: Inguinodynia, is a frequent problem in clinical practice. The need to improve its assessment is obvious. Currently, there is a lack of agreement regarding the appropriate way to evaluate this syndrome. The objective of this paper is to illustrate and propose a modification of the dermatome mapping (DM) tool to improve its efficacy, by combining it, using the analogous verbal numerical scale of pain (VANSP) to identify the affected nerves from the dermatome involved, to objectively evaluate pain intensity and to assess the treatment given.
Methods: We use the analogous verbal numerical scale of pain (VANSP) to mark the patient’s skin, applied to the method of DM described by Álvarez. We ask the patient to characterize the pain intensity with numbers, beginning with one, for the slightest pain, to 10 for the worst possible pain experienced. After the treatment is given, either if it is conservative, surgical, or neural block, we repeat the mapping to evaluate its effectiveness.
Results: We have used this approach since 1997, in several patients with inguinodynia of different etiologies pre- and post-treatment, and it is useful and a more objective evaluation, especially to estimate the result of the treatment given.
Conclusion: Dermatome mapping, marking the patient pain points using the VANSP, can provide relevant information for the diagnosis, treatment, and assessment of management results in patients with inguinodynia.
Keywords: Pain scale, inguinodynia, inguinal hernia, dermatome mapping, verbal analog scale of pain, groin pain, treatment.
Inguinodynia may be a complication after inguinal hernia repair, but it has other causes. It is a problem in terms of its initial assessment and also to evaluate the effectiveness of the treatment. There are many clinical tools to assess inguinodynia, but there is no agreement regarding the best method to evaluate it. Dermatome mapping (DM) has been used for eighteen years as an effective and easy clinical method to evaluate inguinodynia.1-3 It makes it possible to identify if the pain follows a neuropathic pattern, and even to identify the nerves involved.4 But the pain of any kind should not only be identified in terms of medical history, anatomic area, type of pain, clinical presentation, irradiation, and other factors. It also must be objectively evaluated describing the pain intensity referred by the patient, to design the best treatment and to assess the results.5 The objective of this paper is to describe and propose the verbal analog numerical scale of pain (VANSP) applied to the conventional dermatome mapping (DM+VANSP), to improve the efficiency of this clinical tool, assessing the pain intensity as referred by the patient.
Since 1997, we use the DM+VANSP to mark the patient’s skin in a similar way as Álvarez, described in 1998 to identify the affected inguinal nerves. He used color markers and a blunt stimulator to explore the lower abdominal dermatomes below the umbilicus with an “X” or “O” on the patient's skin with marks placed 1cm of distance between each other, with different colors to differentiate between pain or normal sensibility (Figure 1).2,3 We do the test with a blunt point stimulator, 1 centimeter of the distance between each point, from the level of the umbilicus to the upper two-thirds of the genitalia, and from the midline, lateral to the axillary line, each centimeter. Including the anterior surface of the thigh in a grid form asking the patient to characterize the pain intensity with numbers. Marking with number one, for the slightest pain, to 10 for the worst possible pain experienced. If there is no pain, we don’t mark over that point (Figure 2). Until now, we don’t have patients with areas of anesthesia, but if a point with anesthesia is found, it can be marked with the letter “A”. After the treatment is given, either if it is a conservative, surgical, or neural block, we repeat the mapping to evaluate its effectiveness as the patient’s case requires.
We have used this approach in many patients with inguinodynia of different etiologies, pre-and post-treatment. It is helpful as an objective evaluation, mainly to estimate the result of the treatment. To illustrate the usefulness of this clinical tool, we present the following two clinical cases.
Case 1.
A 59-year-old male patient with a family history of a brother died from Hodgkin's lymphoma. He started with right groin pain without irradiation three months before, progressing until he was unable to maintain normal daily activities. He was diagnosed as having orthopedic pain and treated with conventional medication. A month later, he reported progressive pain and a mass growth in the right inguinal region. Physical examination revealed a solid, non-mobile mass in the right groin and pain on palpation over the right inguinal region. Ultrasound confirmed the mass as an enlarged inguinal lymph node. DM+VANSP was performed. The patient referred number two on the dermatome corresponding to the ilioinguinal nerve. The lymph node was removed. Hodgkin's lymphoma was diagnosed, consequently treatment was started. One month after the removal of the lymph node, DM+VANSP was performed again. No inguinal pain was referred by the patient. In this case, we marked with digit 0 the former areas with pain for better comparison (Figure 3).
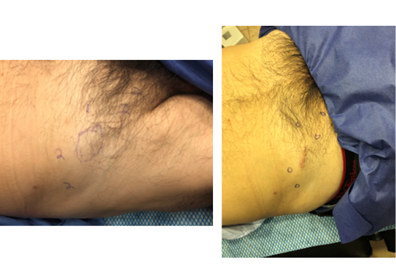
Figure 3 Case 1. DM+VANSP before and after lymph node resection. Former areas with pain were marked with digit 0 post-treatment for better comparison.
Case 2.
A 47-year-old female presented with pain for the last two years in the right inguinal region. She visited several doctors without a definitive diagnosis and different conservative treatments. The pain was progressive until it became constant, preventing her from doing physical activity and persisting even at night. We performed DM+VANSP (Figure 4). Ultrasound, revealed a right inguinal hernia with incarcerated omentum. Laparoscopic TAPP-type inguinal hernia repair with neurolytic block infiltration was performed. The pain decreased until it disappeared almost completely, however, when exercising two weeks after surgery, she hurt himself, presenting a seroma, and DM+VANSP revealed a smaller zone with pain solved with analgesics.
The surface of the skin is divided anatomically into specific areas called dermatomes. A dermatome is an area of skin in which sensory nerves derive from a single spinal nerve root. According to the dermatome affected in patients with groin pain, Álvarez described in 1998 a technique to identify the affected inguinal nerves.2 He used color markers and a blunt stimulator to explore the lower abdominal dermatomes below the umbilicus with an “X” or “O”, on the patient's skin, with marks placed 1cm of distance between each other. He used different colors to differentiate between pain and normal sensibility (Figure 4). Groin pain, also called inguinodynia, is usually an orthopedic problem. Its origin could be diverse: inguinal disruption in sportsmen, hip labral tear, rectus abdominal muscle tear, adductor muscle injury, inguinal floor disruption, spermatic vessel thrombosis, epididymitis. It may be caused by extra-inguinal problems such as lumbar disc herniation, spondylitis, etc.7-11 Also could be caused by an inguinal hernia repair with an incidence near 11%.12 Groin pain syndrome was described by Magee13 in the middle of the last century, to refer the entrapment of the genitofemoral nerve as a result of inguinal or lower abdominal trauma, or after an appendectomy. A few years later, Marsden14 reviewed more than two thousand patients who had undergone inguinal herniorrhaphy; and reported a high frequency of neuritic pain involving the ilioinguinal or iliohypogastric nerves. In 2002, Deysine15 described the inguinal pain syndrome without a hernia, or with a history of previous herniorrhaphy. He associated this problem with a D12-L1 radiculopathy; or neuropathy due to ilioinguinal, iliohypogastric, or the genital branch of the genitofemoral nerves. One of the main challenges to evaluate and treat inguinodynia, is the physician's ability to identify the etiology, whether it is neuropathic or not; and if it is, to identify the nerves involved. Therefore, the optimal management of inguinal pain especially when it is chronic, should begin with a thorough clinical history and physical examination.11,16
Currently, there is a lack of consensus concerning the best way to evaluate this syndrome. There are different methods of assessment. Certain authors consider only the information of its existence. Some series about inguinodynia, include pain characteristics reported by the patient. Others, obtain indirect information derived from questionnaires such as the Quality of Life, Core Outcome Measure Index, McGill, or similar.17-19 Most of the literature about inguinodynia, does not specify the pain intensity. Aasvang used different variables to predict post-hernioplasty pain, using a validated Activity Assessment Scale in patients operated with TAPP (transabdominal preperitoneal) and TEP (total extraperitoneal) techniques, but without identifying pain localization or intensity.20
Ultrasound, computed tomography, and magnetic resonance imaging are also useful to diagnose the etiology of inguinodynia from orthopedic causes, and in patients with inguinal hernia repair. They can identify fibrosis caused by the mesh, hematomas, seromas, or other causes. Assessment can also be done with quantitative sensory testing (QST), which uses various methods to investigate pain by pressure, cold, or pinprick, but they are time-consuming and not applicable to most routine clinical settings. Until now, some guidelines had been proposed to evaluate inguinodynia, but they are based on expert opinions and personal experience, and not on solid high-quality evidence.21
So far, an effective and easy clinical tool to assess the damage of the lumbar plexus nerves involved in the inguinal region is the DM described by Álvarez. The precision of this method was corroborated by the application of evoked potentials. Photos of the mapping can be taken and compared after treatment, and even shared with experts to plan the best management strategy. This mapping system can also identify if the pain doesn’t follow a nerve path, which could be suggestive of another etiology, or if it is a fake symptom for a secondary gain of the patient. But this method leaves aside the assessment of the pain intensity which is pertinent, not only for the initial appraisal but also to evaluate the treatment effectiveness.
The multidimensional nature of pain is well recognized. Therefore, pain intensity should always be considered. There are good quantitative tests to measure and make objective the subjective dimension of pain intensity. The VANSP has been used in dermatome mapping before, but only to describe the general sense of the patient and not to determine the anatomical affected area.23 It has proven to be effective in patients with inguinodynia and has the advantage of its easiness and simplicity. The patient is asked to characterize numerically the pain intensity that he feels as we explore the area. Therefore, we propose to combine the classic dermatome mapping technique, with VANSP, marking the points tested, with numbers from one to ten to evaluate pain intensity. This way we obtain the benefit to evaluate the anatomic dimension, and also the pain intensity in the site at the same time. We only mark the pain points with the number told by the patient, instead of marking the complete area, making easy the assessment. Photos can be taken to compare the patient status pre-and post-treatment.6 It is useful to have a uniform and easy way to evaluate, describe, and document the clinical problem of these patients.24,25
It is also important to consider that chronic pain in some cases, may evolve as severe pain such as hyperalgesia “abnormally increased sensitivity to pain”, or allodynia "pain due to a stimulus that does not normally provoke pain", which must be also be evaluated and documented in the patient record, to better capture the complexity of the pain experience and to treat it properly, and evaluate the results of the treatment given.26,27
Non-operated patients with an inguinal hernia who suffer chronic inguinodynia must have a careful evaluation of the pain, including the pain intensity, to take special measures to treat the pain during hernia repair and not only repairing the hernia, to avoid the surgeon and patient’s frustration if the pain persists after surgery.25 This pre-operative evaluation would be very helpful to measure accurately the results obtained.
A possible drawback when using this technique is the subjective perception of pain which is dependent on individual differences in physiological, emotional, and cognitive states of the patient and not only derived from pain perception, which could differ between mappings.
Dermatome mapping, marking the patient pain points using the VANSP, can provide relevant information for the diagnosis, treatment, and assessment of management results in patients with inguinodynia.
Dr. Alejandro Weber: Conception and design of the investigation. Principal reviewer and investigator. General supervisor.
Dr. Pablo Weber Alvarez: Investigator and reviewer, analysis and interpretation of data.The authors involved in this investigation declare that they don’t have conflict of interest.

©2023 Weber-Sánchez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.