MOJ
eISSN: 2471-139X


Mini Review Volume 7 Issue 3
Centre of Education Progammes, The University of Trinidad and Tobago, Republic of Trinidad and Tobago
Correspondence: William Mollineau, The University of Trinidad and Tobago, Corinth Campus, San Fernando, Republic of Trinidad and Tobago, Tel 1-868-745-1415
Received: April 17, 2020 | Published: May 6, 2020
Citation: Mollineau W. Application of the primary investigations on the anatomical features of the male reproductive system of Dasyprocta species: a mini review. MOJ Anat Physiol. 2020;7(3):54-57. DOI: 10.15406/mojap.2020.07.00290
This mini review examines the major articles published on the anatomy of the male reproductive system of three Dasyprocta species., namely Dasyprocta aguti, Dasyprocta leporine and Dasyprocta prymnolopha. The paper reviews major investigations on the topic and highlights additional research (collection and processing of agouti semen) which used this foundational knowledge set as its catalyst. Future scientific works are recommended based on the existing literature.
Keywords: male agouti, anatomy, reproductive system, assisted reproductive techniques
The agouti is a Neotropical rodent found predominantly in Central and South America, and the Caribbean.1,2 Three of the Dasyprocta species which attached to majority of attention include Dasyprocta aguti, Dasyprocta leporine and Dasyprocta prymnolopha. These edible rodents are delicacy in many of the Neo-tropical territories and evidence indicates where are steady increases in demand for the animal’s meat. Thus, creating a growing need for information on this species and the captive rearing of the agouti affords researchers the opportunity to investigate the ecology of the rodent.3 Interest in farming the animal is increasing and some advocates recommended the domestication of farmed populations.4 Furthermore, the history, trends and benefits of Wildlife farming in Trinidad were also emphasized, giving value to the aforementioned.5 To put into prospective the recent mini avalanche of reviewed articles on the anatomy and physiology of the male’s agouti reproductive system, the majority of which came out of Brazil and some other South American countries, and to a lesser extend Trinidad and Tobago.6,7 This paper focuses on the anatomy of the male reproductive system of three Dasyprocta spp. with the aim to facilitate the reproductive contribution of this initial body of knowledge in other reproductive work on the species.
Testes
The testes of D. leporina and D. prymnolopha are oval-shaped and enclosed in scrotal sacs.8,9 The paired testes were located intra-abdominally as evaginations of the caudoventral abdominal and can be observed as two bulges emerging from under the skin on either side of the inner hind legs (Figure 1 & Table 1).8,10
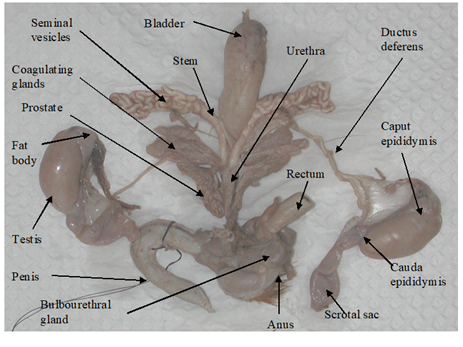
Figure 1 Structure of the reproductive system of a sexually mature male agouti.8
|
Structures |
Species and source |
Length |
Width/Diameter |
Weight |
Volume |
|
Testes |
D. leporina7 |
3.67±0.12cm |
1.67±0.04cm |
5.03±0.52g |
5.39±0.32cm3 |
|
Testicular mass as a % of body mass |
- |
- |
0.48±0.02% |
- |
|
|
Epididymis |
- |
- |
- |
- |
|
|
Ductus deferens |
10.98±0.40cm |
0.14±0.01cm |
- |
- |
|
|
Ampulla |
0.25cm |
- |
- |
||
|
Seminal vesicles (SV) |
4.76±0.15cm |
1.03±0.04cm |
1.28±0.16g |
||
|
SV tubules – Cranial & caudal end, respectively |
- |
0.28±0.16cm 0.16±0.06cm |
- |
- |
|
|
Coagulating glands |
3.10±0.22cm |
1.74±0.12cm |
1.23±0.23g |
- |
|
|
Prostate glands (PG) |
3.50±0.12cm |
1.10±0.02cm |
0.99±0.41g |
- |
|
|
Lobes for PG |
1.27±0.07cm |
0.11±0.04cm |
- |
- |
|
|
Bulbourethral glands |
1.47±0.01cm |
1.65±0.11cm |
1.27±0.16g |
- |
|
|
Penis |
9.90±0.43cm |
1.60±0.17cm |
4.72±0.25g |
- |
|
|
Glans penis |
3.18±0.22cm |
0.80±0.18cm |
1.80±0.04 g |
- |
|
|
Corpus penis |
6.72±0.43cm |
0.76±0.03cm |
2.94±0.25g |
- |
|
|
Keratinaceous styles |
1.20±0.03cm |
w0.18±0.01cm |
- |
- |
|
|
os penis |
2.5cm |
w0.66cm n0.29cm |
- |
- |
|
|
Lateral penile cartilage |
1.6cm |
w0.20cm n0.16cm |
- |
- |
|
Table 1 Measurements
w wider end
n narrower end
-information not available
Epididymis
Epididymis similarities were described for D. leporina, D. aguti and D. prymnolopha. The coiled tubular structure adheres to the testes and runs the entire length. The caput (head) epididymis is enclosed by fat and appears as the least convoluted segment of this organ, while the coiling intensifies as the tubular organ approaches the cauda epididymis (Figure 1 & Table 1).8–11
Ductus deferens
These paired ducts are the continuation of the cauda epididymis which uncoil after leaving the epididymis. These ducts are thick-walled tubes which terminate in the urethra.8,10 As the duct approached the urethral end the diameter of each duct expands to form the ampulla (Figure 1 & Table 1).8
Accessory sex glands
The accessory sex glands of the sexually mature male members of D. leporina,8,12 and D. prymnolopha10,13 include the seminal vesicles (SV), the coagulating glands (CG), the prostate glands (PG) and the bulbourethral glands (BG) and are individually encased in a thin transparent facial sheath.8 These glands are attached to the ductus deferens end of the urethra by a short stock/stem (Figure 1).
The SV (Figure 1 & Table 1) of D. leporina and D. prymnolopha are branched structures that appear as tightly coiled tubular masses. An ejaculatory duct opening at the dorsal end enters the urethra with that of the ductus deferens in close proximity.8,13
The CG of the agouti are placed to each side of the urethra and composed of lobes that emerge from the base close to the SV.13 These glands are composed of approximately 56 finger-like structures with some degree of branching.8 On each side of the urethra the CG are closely adhered to the stem and main branch of the SV.
The PG showed two equal lobes, the ventral lobes are located laterally to the urethra and the dorsal lobes composed of convoluted tubules. The lobes are composed of approximately 45 tubules (Figure 1 & Table 1).8
The paired BG of D. prymnolopha were described as rounded organs, lateromedially compressed, and covered by a thin layer of conjunctive and muscular tissue13 while those of D. leporina were bean-shaped.8 In both species the BG are approximately dorsolaterally to the rectum and dorsocranially to the anal sphincter muscles and a single duct penetrates into the urethra before the formation of the penis bulb (Figure 1 & Table 1).8,13
Penis
In D. aguti, D. leporina and D. prymnolopha the corpus penis is attached by the ischiocavernosus muscle towards the ischiatic arch and the glans penis is located in the prepuce. The retracted penis lies on the ventral side of the pubis symphysis below the skin, caudally directed and is U-shaped (Figure 1).8,9 The penis of D. prymnolopha was characterised as fibrocavernous with large amounts of the keratized structures in the their apex9. The glans penis of D. leporina is covered by penile spines (spike-like studs) and those studded on the head are much smaller than those embedded on the shaft of the glans pens.8,9 There is a paddle like bone, the os penis (osteomusculocavernous) below the dorsal surface of D. leporina glans penis (Figure 2).
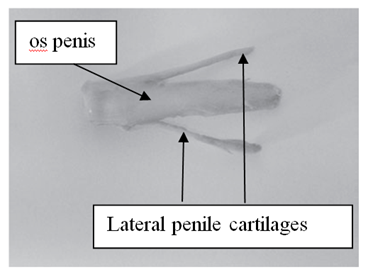
Figure 2 Os penis and lateral penile cartilages of Dasyprocta leporina.8
Two bones which are tapered into needle points (keratinaceous styles) are located inside the intromittent sac (Figure 3 & Table 1). These keratinaceous styles are slightly curved downwards. The crainal ends of these keratinaceous styles are attached to the caudal end of the intromittent sac. On each lateral side of the glans penis there is a slender structure, the lateral penile cartilage. Its dorsal edge is attached to the lateral side of the glans penis, while its ventral side is free. Thus, this lateral penile structure, which is flexible, can be raised laterally (Figure 2). This cartilage is attached to the cartilage that joints the caudal corpora cavernosa and the cranial os penis by a muscle on each lateral ventral end.8,9 The morphological changed during the four stage of erection were described for D. leporina (Figure 3).14
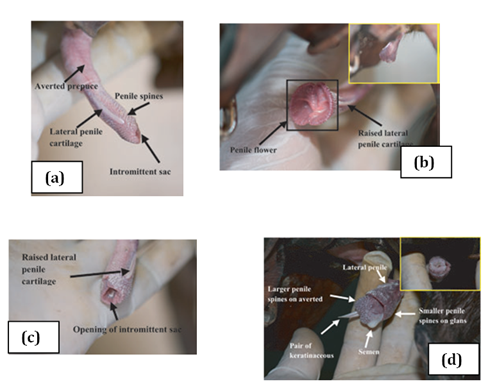
Figure 3 Agouti penile erection: (a) stage 1, penis protrudes out of the preputial orifice; (b) stage 2, lateral penile cartilages raising vertically and spread laterally; (c) stage 3, formation of the penile flower before the protrusion of the averted intromittent sac; (d) stage 4, complete erection and ejaculation.14
There were similarities in the morphology of the male agouti’s reproductive system amongst D. aguti.11,15,16 D. leporina8,12,14 and D. prymnolopha.10,13 Dimensions were published for the various structures comprising the male reproductive system for D. leporina (Table 1). However, corresponding statistics were not available for D. aguti and D. prymnolopha. The plastid "U" flexure of the species penis was comparable for the three species. The various stimulatory and anchorage appendages (penile spikes, os, keratinaceous styles, lateral penile cartilages)8,9 identified with the agouti’s penis suggest that the female of the species may possible display reflect ovulation, at least in some part. However, some reports indicated that spontaneous ovulation seems to occur after the progesterone peak. These reports recommended more detailed research to generate additional evidence on ovulation in D. prymnolopha.17 Further meaningful comparisons and or contrasts are not possible because of the sporadic reporting on these three species. Generally, the evidence revealed through the published literature on the male reproductive system suggests that close relationships do exist, such as the genetic classification of the Dasyprocta species.18 However, definite conformation of any genetic relationships amongst the presently define Dasyprocta species may require the intervention of DNA technology.
These morphological investigations were important because they established the foundations for further research such as identifying to unset of sexual maturity in the male agoutis.11,15,16 Therefore, it was and is possible to select specific animals based on their age15 for breeding and other reproductive trials. Semen collection7,19 analysis,20 extension, preservation,21 and spermatogenesis10 in the species have already begun. After the processing of semen the uncharted area of artificial insemination (AI) in the agouti is possible as the foundational pool of knowledge to initiate the application of this reproductive technique is available. Namely, anatomy of the male and female3 reproductive system have increased the limited body of knowledge on the species. Furthermore, the estrous cycle,22,23 related hormonal activity24,25 and stimulatory effect of the presence of the male agouti on the onset of female puberty17 will assist in the application of assisted reproductive techniques (ART) in the species. Technicians would be guided by the anatomy of the female’s reproductive tract for the intrusion of probes and other invasive instruments to deposit semen samples at the precise point of fertilization. Other ART such as estrus synchronization may also from a valuable part of the domestication of the Dasyprocta spp.4,26
The anatomy of the male reproductive systems of Dasyprocta aguti, Dasyprocta leporine and Dasyprocta prymnolopha present many morphological similarities. This knowledge was instrumental in other investigations such as identifying the age range for sexual maturity in the male agoutis, semen collection, analysis and processing, preservation, stages of spermatogenesis and stages of erection of the agouti’s penis. Further useful research may involve artificial insemination and ARTs.
None.
The authors declare there are no conflicts of interest.
None.

©2020 Mollineau. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.