MOJ
eISSN: 2471-139X


Research Article Volume 5 Issue 2
Department of Anatomy, King Abdulaziz University, Saudi Arabia
Correspondence: Ghada A Abdel Hamid, Associate professor, Department of Anatomy, King Abdulaziz University, Saudi Arabia
Received: February 21, 2018 | Published: April 9, 2018
Citation: Abdel-Hamid GA. Ameliorative effect of vitamin C on nicotine-induced histological and ultrastructural changes in zona fasciculata in albino rats. MOJ Anat & Physiol. 2018;5(2):120-125. DOI: 10.15406/mojap.2018.05.00175
Nicotine is the main toxic constituent in cigarettes and vitamin C is an important antioxidant agent. This study investigated the possible ameliorative effect of vitamin C on nicotine-induced histological and ultrastructure changes in zona fasciculata in rats. Sixty adult male albino rats were distributed into 3 groups (20 rats each): Group I, control group received subcutaneous injection of saline daily for 8 weeks. Group II nicotine-treated group, rats received subcutaneous injection of nicotine (0.7 mg/kg body weight) daily for 8 weeks. Group III (nicotine+vitamin C group), rats received vitamin C (100 mg/kg body weight daily) through intragastric tube. Samples from adrenal cortex were prepared for light (hematoxylin & eosin and toluidine blue staining) and electron microscopic examination. Nicotine resulted in histopathological changes in zona fasciculata as cells had cytoplasmic degeneration with increased lipid contents, significant increase in total adrenal cortical thickness and zona fasciculate thickness. Nicotine induced ultrastructure changes in zona fasciculata cells and a significant increase in number and volume of lipid droplets, degenerated mitochondria, decreased the surface area of mitochondrial cristae, the volume of mitochondrial compartment and increased significantly the volume of lipid droplet compartment. These histopathological and ultrastructure changes in zona fasciculata cells due to nicotine exposure, were reduced by vitamin C supplementation. In conclusion, this study provided evidence that the harmful histological and ultrastructure changes of nicotine on zona fasciculata in rats were ameliorated by vitamin C intake.
Keywords: fasciculata, histological, nicotine exposure, rats, vitamin C supplementation, ultrastructural change
The harmful effects of tobacco products and smoking cigarette are well known. There is significant scientific evidence of increased morbidity and mortality in the developed and developing countries due to intake of tobacco products. Nicotine is the main component in tobacco and is accountable for its addiction.1 Moreover, it is the main pharmacologically active material in cigarette smoke. Smoking influences a lot of metabolic procedures in the body due to secretion of hormones.2
Adrenal glands secrete hormones, including cortisol. Cigarette smoking changes the endogenous steroid hormones levels. An increase in circulating cortisol level has been detected after smoking.3 Additionally, Cortisol levels descent considerably when people give up smoking particularly through the initial withdrawal process. Furthermore, smoking leads to an increase in levels of dehydroepiandrosterone sulfate, androstenedione,4 17-hydroxyprogesterone and dehydroepiandrosterone in male smokers.5 In experimental studies, cortisol modifies some of the behavioral and physiological effects of nicotine6 and the responsiveness of animals to nicotine.7
Regarding the harmful effect of nicotine on zona fasciculata, earlier studies, revealed that subcutaneous injection of nicotine induced damage in normal histological architecture and ultrastructure of cells in zona fasciculata. The most remarkable changes were swelling of cells, obvious cytoplasmic vacuolation, degeneration of mitochondria and a striking increase in lipid deposition.8,9
Vitamin C (Ascorbic acid) is a crucial antioxidant which is water-soluble in human serum. It acts as a biological antioxidant, decreasing the oxidative properties of lethal substances. Smokers have low concentrations of vitamin C in comparison to the non-smokers.10 Vitamin C has the ability to scavenge free radicals and form a strong defence line in contradiction of the reactive oxygen species (ROS) that induce cellular damage.11
Co-administration of vitamin C with nicotine significantly decreased malondialdehyde, liver enzymes and high glutathione level in kidney and liver tissue. Moreover, Vitamin C co-administration increased significantly the proliferating ability of renal and hepatic tissue.12 Previous studies investigated the ameliorative effect of vitamin C to reduce oxidative stress that resulted from exposure to nicotine on biochemical basis.13‒15 On the other hand, little attention has been given to possible protective effects of vitamin C with nicotine administration on histological and ultrastructure levels. Therefore, the aim of this study is to determine the histopathological and ultrastructure effects of nicotine administration on zona fasciculata in rats’ adrenal cortex and probably the protective effects of Vitamin C.
Animals
Sixty adult male albino rats (Wistar) weighing 250–275 grams were accommodated in the animal facility at King Fahd Medical Research Center (Jeddah, Saudi Arabia). The study was conducted under the ethical rules and guidelines of the Canadian Council on Animal Care. The rats were kept at 22–24°C temperature, with 55% relative humidity with 12-h light/12-h dark intervals, beginning at 6 a.m. Animals had food and water ad libitum and were acclimatized for two weeks before the start of the experiment.
Chemicals
Nicotine 100% concentration, glass bottle (25 ml) was obtained from Sigma Chemical Co. (St. Louis, MO, USA) and Merck (Darmstadt, Germany). The solutions were prepared with double distilled water.
Experimental design
Sixty male adult albino rats were divided into three groups (20 rats each). Group I served as control group (C) that received subcutaneous injection of saline daily for 8 weeks. Group II, nicotine group rats received nicotine (0.7 mg/kg body weight), daily with subcutaneous injection for 8 weeks.16 Group III (Nicotine+vitamin C group), rats received vitamin C (100 mg/kg body weight daily) through intragastric tube, before subcutaneous injection of nicotine (0.7 mg/kg body weight).17 The dose of nicotine that was used in this study (0.7 mg/kg/day) resulted in plasma levels of 120 ng/ml for cotinine and 30 ng/ml for nicotine which was equivalent to the values in a human smoker consuming 10–20 cigarettes per day.18
At the end of the experiment (8weeks), all the rats were sacrificed with sodium pentobarbital. The abdominal cavity was opened and adrenal glands (right &left) were immediately removed and weighed. One of them was fixed in buffer formalin for paraffin fixation to be stained by hematoxylin and eosin.19 Another one was fixed in 3% glutaraldehyde and 1% paraformaldehyde in 0.1 mol/l PSB (pH 7.4) for 3 hours and fixed in 1% osmium tetroxide in the same buffer for 2 hours. Afterwards, the samples were dehydrated with alcohol in ascending grades and implanted in epoxy resin mixture. 1 μm thickness of semithin sections were stained with toluidine blue.20 The ultrathin sections were stained with uranyl acetate and lead citrate. Sections were examined by the transmission electron microscope (JEM-2000EX; Jeol, Tokyo, Japan) at King Fahd Medical Research Center.
Morphometric measurements
The total thickness of adrenal cortex and thickness of the zona fasciculata in H&E stained sections were measured by using the image analyzer computer system Leica Qwin 500 (Leica, Cambridge, UK). Morphometric measurements for cell organelles were made using electron micrographs, at magnification of 14, 000. The mitochondrial compartment volume (μm3/cells) and the volume of lipid droplet compartment (μm3/cells) in zona fasciculata were measured by using a square lattice test system of type A.21
Statistical analysis
Weights of rats’ body, adrenal, serum cortisol level, morphometric measurements were estimated as mean value ±standard deviation and using the Student t-test. P<0.05 was considered as the significant level of acceptance.22
Effect of nicotine and vitamin C on body weight and adrenal weight
Body weights of rats, which received nicotine (0.7 mg/kg body weight) in group II for 8 weeks significantly decreased (P<0.05) in comparison to the control and nicotine+vitamin C groups. Adrenal weight was increased significantly (P<0.05) in nicotine group when compared with other groups (Figure 1).
Light microscopic results
Control group showed normal histological structure of the zona fasciculata in the adrenal cortex as observed in hematoxylin and eosin. The zona fasciculata cells were arranged in long straight cords separated by blood sinusoids. These cells were large with deeply stained rounded nuclei (Figure 2A). In group II that received nicotine, zona fasciculata cells revealed swelling and distinct increase in cytoplasmic degeneration. Particular cells appeared with faint staining in nuclei and others were stained dark (Figure 2B). Regarding group III which received nicotine with vitamin C, showed significant decrease in the histological changes that were observed in the group II due to receiving nicotine. Zona fasciculata cells were showing findings almost similar to the control group (Figure 2C). Nicotine administration subcutaneously (0.7 mg/kg body weight) daily for 8 weeks resulted in significant increase (P<0.05) in total cortical thickness of the adrenal cortex and zona fasciculata thickness in comparison with the control and nicotine + vitamin C groups (Figure 3).
Semithin sections of the zona fasciculata stained with toluidine blue in the control group showed normal polyhedral cells with round nuclei. The cells had deeply stained nucleoli, containing lipid droplets (Figure 4A). In nicotine group, the cells had stained nuclei and marked an increase in the lipid droplets (Figure 4B). The zona fasciculata in nicotine + vitamin C group showed a reduction in the lipid droplets with dilated blood sinusoids, similar to the control group findings (Figure 4C).
Electron microscopic findings
The ultrathin sections in Control group, showed the normal ultrastructure of the zona fasciculata cells. The nucleus had prominent nucleolus and was euchromatic. The mitochondria were variable in size and spherical with dense vesicular cristae (Figure 5A) (Figure 6A). The zona fasciculata in nicotine group showed an increase in lipid droplets. Nuclei had condensed chromatin on their irregular outlines (Figure 5B) (Figure 6B). Several lipid droplets with dark (dense) areas were detected (Figure 6C) (Figure 6D). In addition, several degenerated mitochondria and appearance of (myelin figure) which consists of concentric spirals of membranes were noticed (Figure 6C) (Figure 6E). Electron microscopic findings of the zona fasciculata cells in rats which received Vitamin C plus nicotine showed nearly normal structure with euchromatic nucleus and a few lipid droplets. Mitochondria were variable in size with dense vesicular cristae (Figure 6F).
Daily dosage of subcutaneous nicotine (0.7 mg/kg body weight) for 8 weeks significantly decreased (P<0.05) the surface area of mitochondrial cristae and the volume of mitochondrial compartment. On the other hand, nicotine increased the (P<0.05) volume of lipid droplet compartment significantly (Figure 7).
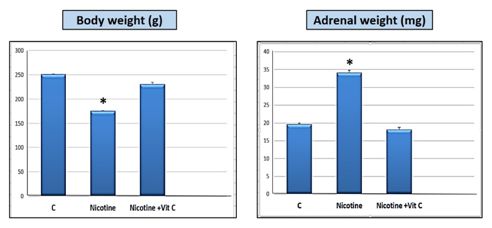
Figure 1Nicotine is significantly (*P<0.05) decreased the rats body weight and increased adrenal weight in comparison with both control (C) and nicotine+ vitamin C groups.
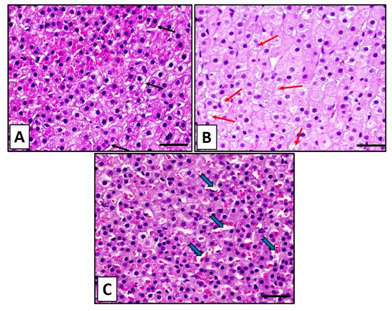
Figure 2Hematoxylin and eosin staining sections for the zona fasiculata in adrenal cortex of control group (A) showing well-arranged cell cords of zona fasciculate separated by blood sinusoids (black arrows). (B): The zona fasiculata in nicotine group shows swelling of the cells with degeneration (red arrows). (C): In (nicotine+vitamin C) shows findings as control group with dilated sinusoids (blue arrows). Scale bar: 50 µm.
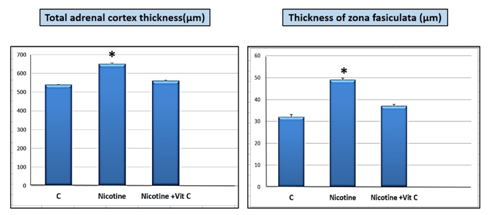
Figure 3 Nicotine is significantly (*P<0.05) increased both the total adrenal cortex thickness and the thickness of zona fasiculata in comparison with both control (C) and nicotine+ vitamin C groups.
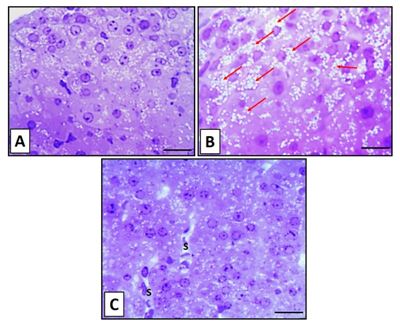
Figure 4 Toluidine staining sections for the zona fasiculata in adrenal cortex of control group (A) showing normal structure of cells in the zona fasciculate with vesicular nuclei and lipid droplets. An obvious increase of lipid droplets (red arrows) are seen in nicotine-treated group (B). Almost, normal structure in cells of the zona fasiculata in nicotine+vitamin C group (C) with dilated blood sinusoids (S). Scale bar: 50 µm.
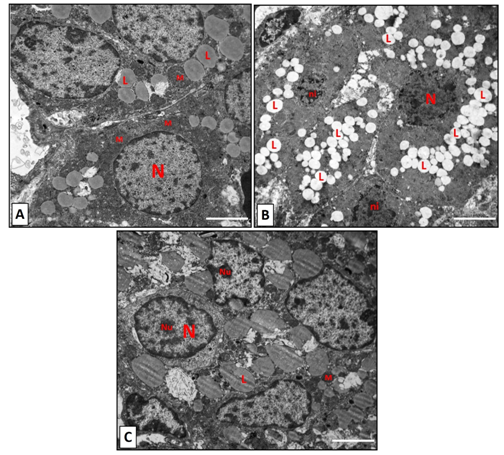
Figure 5 Electron microscopic graphs for zona fasiculata. (A): control group, showing a multiple nuclei that are euchromatic (N). The mitochondria (M) are variable in size spherical in shape. Lipid droplet with different size (L) are seen. (B): The nicotine group showing small nuclei with irregular outlines with condensed chromatin (ni). Obvious increase in the lipid droplets (L) are noticed. (C): the nicotine with vitamin C showing nuclei with variable size with prominent nucleoli (Nu). Dense mitochondria (M) and lipid droplets (L) with variable size are seen. Scale bar: 2 µm.
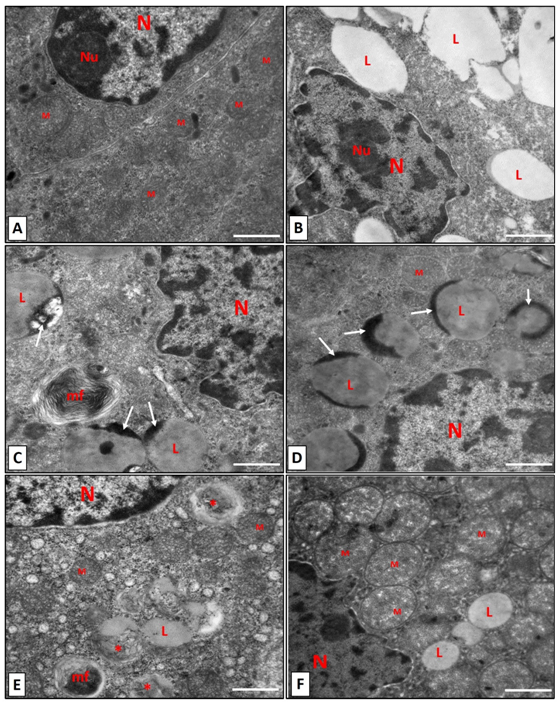
Figure 6 Higher magnification for zona fasiculata cells. (A): control group, showing a nucleus (N) with prominent dense nucleolus (Nu). Multiple mitochondria (M) with variable size are seen. (B-E) the zona fasiculata of nicotine group showing, irregular nuclear outlines with condensed nucleolus (Nu) and several lipid droplet with different size (L) are seen in (B). Dense areas are noticed in lipid droplets (white arrows) in (C,D). Myelin figure near lipid droplets is detected in (C, E). Degenerated mitochondria (*) beside normal one (M) is noticed in (E). In (F): Normal structure of mitochondria (M) and nucleus in nicotine+vitamin C group. Scale bar: 500 nm.
Nicotine is the main harmful constituents of a cigarette.23 Cigarette smoking has numerous effects on endocrine glands as pituitary, adrenal, thyroid, ovarian and testicular function.24 Additionally, it induces widespread disturbance in hormone levels that secretes from adrenal cortical.25 Nicotine intake in rats disturbed the antioxidant defence in rats, increased the lipid peroxidation and reduced the antioxidants in tissues.26 The present study was conducted to investigate the possible ameliorative effect of vitamin C against the harmful effect of nicotine on ultrastructure changes in zona fasciculata in rats’ adrenal cortex.
The results revealed that administration of nicotine (0.7 mg/kg body weight) daily for 8 weeks significantly decreased rats’ body weight. On the other hand, nicotine intake increased the adrenal weight significantly. This finding was supported by a previous study that indicated that nicotine induced accumulation of lipid droplets in zona fasciculata cells that resulted in an increase in adrenal weight.8,27
Regarding histopathological changes due to nicotine exposure, zona fasciculata cells showed swelling, cytoplasmic degeneration with increased lipid contents. These changes explained the significant increase in the total adrenal cortical thickness and zona fasciculata thickness in this study. Similar histopathological changes have been documented formerly.8,9 Swelling and increased accumulation of lipid droplets due to administration of nicotine resulted an increase in the thickness of zona fasciculata and consequently, an increase in the total thickness of adrenal cortex.
Nicotine resulted in ultrastructure changes in zona fasciculata cells as well as a significant increase in number and volume of lipid droplets, degenerated mitochondria and presence of myelin figure. These findings were confirmed by morphometric measurements which revealed that, nicotine significantly decreased the surface area of mitochondrial cristae, the volume of mitochondrial compartment and increased significantly the volume of lipid droplet compartment. Earlier studies are in agreement with the findings of this research as they validated an increase of lipid droplets and presence of vacuolation in the zona fasciculata cells due to exposure to nicotine which impaired the glucocorticoids synthesis which occurred in zona fasciculata cells.8,9 This occurred due to cytochrome P450 enzymes disruption. Consequently, cholesterol biosynthesis was inhibited that lead to lipid droplets accumulation in zona fasciculata cells. Furthermore, steroid synthesis depended on integrity of mitochondria to be completed.28 Accumulation of cholesterol within the mitochondria resulted in its cavitation and degeneration and subsequently, steroid synthesis was decreased.29
Cell toxicity caused by nicotine in the current study was due to reactive oxygen species. As nicotine induced oxidative stress both in vivo and in vitro, increased free radical and lipid peroxidation was reported.30‒32 Reactive oxygen species induced peroxidation of phospholipids in cell membrane that altered membrane fluidity and resulted in damage of cellular integrity. Reactive oxygen species attacked cellular DNA and lysosomes, leading to cellular damage and death.33 The mitochondria, is more sensitive to reactive oxygen species. Therefore, it was the most affected organelle by nicotine administration that induced degeneration of mitochondria, in this study.
In the present study, vitamin C (100 mg/kg body weight daily) ameliorated the harmful effects of nicotine as it restores body weight, adrenal weight, total thickness of adrenal cortex, and thickness of zona fasciculata. Furthermore, vitamin C significantly improved the histopathological and ultrastructure changes in zona fasciculata cells due to nicotine exposure. Nicotine administration also affected the zona fasciculata cells through increased reactive oxygen species and lipid peroxidation. These ameliorating effects of vitamin C in the current study endorsed its ability to decrease lipid peroxidation products level.34 It reduced the reactive oxygen species to inhibit propagation of lipid peroxidation.35 Additionally, vitamin C, react with hydroxyl radical, superoxide and singlet oxygen. Consequently, it reduces the tocopherol radical back to α-tocopherol.36 Moreover, vitamin C can protect against lipid peroxidation by scavenging of reactive oxygen species.37
Subcutaneous administration of nicotine (0.7 mg/kg body weight) daily for 8 weeks induced morphological, histological and ultrastructure changes of the zona fasciculata cells. These changes are mostly due to the oxidative stress and reactive oxygen species that resulted from the administration of nicotine. Oral administration of Vitamin C (100 mg/kg body weight) with nicotine ameliorated these changes. The results suggest to discontinue smoking because of its harmful effects, and Vitamin C as an antioxidant should be given to minimize the hazardous effects of nicotine in smokers.
None.
The authors declare that they have no conflicts of interest regarding this study.

©2018 Abdel-Hamid. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.