MOJ
eISSN: 2471-139X


Research Article Volume 5 Issue 2
Department of Human Anatomy, University of Nairobi, Kenya
Correspondence: Johnstone M Muthoka, Department of Human Anatomy, University of Nairobi, PO Box 30197, 00100, Nairobi, Kenya, Tel +254 724 814 435
Received: June 29, 2017 | Published: March 6, 2018
Citation: Muthoka JM, Hassanali J, Malek AA, et al. Sex differences in histomorphology of the human carotid body. MOJ Anat & Physiol. 2018;5(2):74-78. DOI: 10.15406/mojap.2018.05.00167
Knowledge of sex differences in the histomorphology of the carotid body is important in understanding observed differences in respiratory function and disorders. There are few studies on sex differences in the carotid body. This study therefore aimed at describing these differences in the histomorphology features of the human carotid body.
Thirty six random carotid bodies from cardiovascular disease free individuals (20 male, 16 female) obtained during autopsy at the Department of Human Anatomy, University of Nairobi were studied. Specimens obtained within 48 hours of death were fixed in 10% formaldehyde solution and processed routinely for paraffin embedding. Seven micron thick serial sections were stained with H&E, Mason’s trichrome and examined with light microscope. Stereology was done to determine area occupied by parenchyma, stroma and vasculature. Data were analyzed using SPSS version 13.0. The student’s t test was used to compare sex differences. The data are represented using tables and macrographs.
The human carotid body is highly cellular with two varieties of cells namely; chief and sustentacular. It is heavily vascularised and densely innervated. Females showed higher concentration of chief cells, more profuse vascularization and denser innervation when compared with their aged matched male counterparts. The aging changes characterized by fibrosis, cellular degeneration vascular and neural attenuation are less severe in females.
Sex histomorphological differences in the human carotid body characterized by higher population of chief cells, density of vascularity, innervation and blunted age related morphological attenuation underpin differences in its function and disease.
Keywords: gender differences, carotid body, cells, vascularization
Knowledge of sex differences in the histomorphology of the human carotid body (HCB) is important in explaining the differences in respiratory function, responses and disease predisposition.1,2 Functionally, there are sex differences in the ventilatory response to hypoxia (VRH) and hypercapnia,1,3‒5 response in ventilatory control under acute or chronic hypoxic stimulation;6 morphine induced ventilatory depression,7 ventilatory roll off and post hypoxia facilitation8 and carotid body based control of ventilation.9 Further, ovarian hormones affect CB function.10 Regarding disease predisposition, hypoxia–mediated ventilatory diseases like sudden infant death syndrome, mountain sickness, high attitude pulmonary edema and sleep apnoea occur more commonly in males and post-menopausal females.10‒12 There are also sex differences in symptoms, diagnosis and consequences of obstructive sleep apnoea.13 There are, however, few studies on gender comparison of CB, with some denying variability.14
This study therefore investigated the histomorphological features of CB in males and females with a view to defining any sex differences in this organ.
Materials for this study comprised human carotid bodies obtained within 48 hours of death during autopsy at the Department of Human Anatomy, University of Nairobi. Ethical approval was obtained from the Kenyatta National Hospital Ethics Review Committee. Cases with history of cardiovascular disease such as hypertension, stroke, myocardial infarction, peripheral vascular disease and diabetes mellitus were excluded as were those with history of cardiovascular disease risk factors namely cigarette smoking obesity, hyperlipidemia and those who showed features of carotid atherosclerosis. Thirty six CB specimens, categorized under sex and age-sets as shown in Table 1 were studied.
Whole carotid bodies, as well as carotid bifurcation were fixed in 10% formaldehyde for 12 hours and dehydrated in increasing alcohol series of 70%, 80%, 90%, 95% and 100% ethanol for an hour in each grade. They were cleared using toluene and infiltrated in molten wax for 12 hours. Each carotid body was embedded in paraffin wax and oriented to its largest diameter, parallel to the plane of section. Seven micron thick serial sections were made using a sledge microtome. Five representative sections, selected from upper, middle and lower portions of the carotid body, were stained with Hematoxylin/Eosin and Mason’s trichrome to demonstrate cells, nerves, connective and vascular tissue.
The slides were examined using a light microscope at magnifications of X 40, X100 and X 400. Representative micrographs of sections within the age groups were taken using a high resolution digital camera.
For stereological study, sections from the proximal, middle and distal parts of each carotid body, according to sex and age group were used. A magnified image (X 1000) of each slide was projected from the microscope onto a television screen and a transparency with a point counting grid (2 cm2) placed on it. The area occupied by parenchyma, stroma and vasculature was determined and percentages obtained recorded. Data was analyzed using SPSS 13.0. The student’s t test was used to compare gender- based differences in mean areas. The results are presented in macrographs and a table.
Age-set |
Age-group |
Number |
Young |
(0-25 yrs) |
M- 5 |
F- 5 |
||
Middle |
(26-59 yrs) |
M- 11 |
F – 6 |
||
Old |
(60 yrs and above) |
M- 4 |
F- 5 |
Table 1 Carotid body sample by age
The HCB was a highly cellular, richly vascularized and densely innervated organ. It was surrounded by a connective tissue capsule with septae that lobulated the organ. These septa contained glomic arterioles and nerve bundles (Figure 1).
Within lobes of the HCB, cells were arranged in clusters with a variable number of type I glomic cells (Chief cells) surrounded by type II sustentacular cells (Figure 2A) (Figure 2B). Type I cells had a rounded contour and contained a large eccentric nucleus. They appeared as dark, light or progenitor subtypes and had numerous cytoplasmic extensions. The staining characteristic of chief cell nuclei designates them as either dark or light varieties. They were both euchromatic with little cytoplasm and exhibit features of protein synthesizing cells. Progenitor cells had small heterochromatic nuclei, little cytoplasm and were scattered within each cluster.
Sustentacular cells were spindle-shaped with faintly eosinophilic cytoplasm and elongated nuclei which were broader than the tapered nuclei of fibrocytes or crescentic nuclei of capillary endothelial cells.
There were sex differences in the organization of glomic and neurovascular tissues. In the male, glomic tissue was compact and surrounded by large blood vessels and nerves with little ramification in septa (Figure 3A). In the female, glomic tissue was diffuse in scattered lobules that were intensely supplied by numerous blood vessels and nerves (Figure 3B).
Within the lobes, capillaries and nerve branches were limited to septa with minimal parenchymal supply in males (Figure 3C, 3E & 3G). In the females, on the other hand, there was extensive supply to the cells within clusters with numerous capillaries being seen in the parenchyma (Figure 3D, 3F & 3H).
In the cellular clusters, the populations of dark and light staining chief cells were similar in the male specimens studied and only the progenitor cells seemed to decline with age (Figure 3C) (Figure 3E). In the females, however, there was a prominence of light staining chief cells with much fewer dark and progenitor varieties (Figure 3D) (Figure 3F).
Within the elderly, degenerative changes in glomic tissue showed the same pattern for both males and females with attendant connective tissue infiltration. Vascularization was, however, better preserved in the females (Figure 3G) (Figure 3H).
The average area of the HCB was determined for both sexes in the three age groups. It was slightly higher in the females for each age group and increased with age. The percentage of glomic tissue was higher in females for all age groups as was that of vascular tissue. Connective tissue content was however higher in male specimens for all age groups (Table 2).
Using the student’s-t test the differences in mean area of the HCB between males and females was not statistically significant (two-tailed P value: 0.7969).
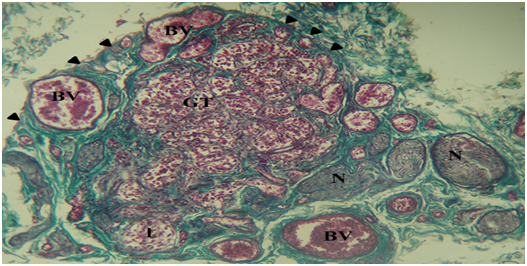
Figure 1 Photomicrograph of a section of the carotid body and surrounding structures. The cellular glomic tissue (GT) is lobulated (l) and surrounded by a connective tissue capsule (arrowheads). It is abundantly supplied by nerve bundles (N) and blood vessels (BV). Mason’s trichrome stain x100.
Figure 2 Photomicrographs showing clusters of cells in the carotid body.
A. Spindle-shaped sustentacular cells (s) surround groups of chief cells, dark (d), light (l) and progenitor (p) varieties. H&E X400
B. Note the proximity of blood vessel (BV) and nerve (N) to a cluster of cells. Mason’s Trichrome X400
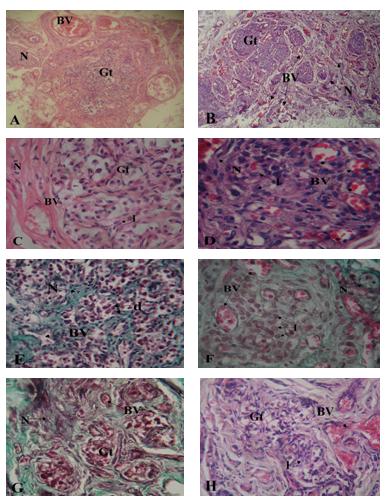
Figure 3A-3H Photomicrographs showing sections of carotid bodies of males and females from different age groups. (A – D, H/E; E, F, G: Masson’s Trichome x 400)
A. 2 yr old male. Blood vessels (BV) and nerves (N) are located around glomic tissue (Gt). X100
B. 3 yr old female specimen, note blood vessels (BV) and nerves (N) within the glomic tissue (Gt). X100.
C. 17 yr old male. Note blood vessel (BV) and nerve (N) located in a septum at the periphery of glomic tissue (Gt). There are few light staining chief cells (l). X400
D. 22 yr old female, note blood vessels (BV) and nerve (N) closely located amid clusters of light staining chief cells (l) with few dark (d) and progenitor (p) chief cells. X400
E. 42 yr old male showing blood vessel (BV) and nerve (N) located in connective tissue septa (s). Cell clusters are mainly composed of dark (d) chief cells. X400
F. 40 yr old female. Note the great number of light staining chief cells (l), blood vessels (BV) and nerves (N) located within glomic tissue (Gt).X400
G. 75 yr old male. Lobules are degenerated and fibrosed. Blood vessels and nerves are few and scanty. X400
H. 82 yr old female. Fibrotic changes are similar to those seen in the male but with presence of few light cells (l). Blood supply is however well maintained (BV). X400
Age group |
Average area (cm2) |
Average percentage of tissues (%) |
||||||
Glomic |
Vascular |
Connective |
||||||
Male |
Female |
Male |
Female |
Male |
Female |
Male |
Female |
|
0-25 yr |
0.36±0.05 |
0.37±0.05 |
70.2 |
75.7 |
8.2 |
12.3 |
21.6 |
12 |
26-59 yr |
1.13±0.08 |
1.15±0.07 |
58.3 |
64.5 |
10.4 |
14.7 |
31.3 |
20.8 |
60 yr + |
1.79±0.08 |
1.84±0.08 |
25.2 |
30.6 |
14 |
21.2 |
60.8 |
48.2 |
Table 2 Average area and percentages tissues found in the carotid body for different age groups in males and females
The parenchyma of the carotid body was composed of clusters comprising chief and sustentacular cells. It was richly vascularized and innervated. This basic structure is similar to that previously reported in domestic fowl,15 human,16 rabbit17 and rat2. This suggests that cytoarchitecture of the CB is preserved across species, probably due to its role as the main arterial chemoreceptor in higher vertebrates.16 The current study recorded major gender differences in vascularization, cellular density and age related attenuation in the CB.
Females had more dense vascularization and innervation than males. This is at variance with reports that there are no structural differences between male and female HCB.14 The high vascularity of HCB is essential to its function for detecting changes in the composition of blood.18‒20
Blood capillaries and sinusoids accompanied by nerve radicals within the cellular lobules ensure maximal contact of the cells with blood and nerve endings. The higher vascularity and innervation of the CB in females may constitute the structural basis for the higher functional capacity of the female especially in ventilatory roll off and post hypoxia facilitation,8 morphine induced ventilatory depression7 and higher ventilatory response to hypoxia.3,4
Pertinent to this is the suggestion that intra organ hemodynamics play a role in the process of chemoreception.2 Studies on the VRH in mice and human subjects show an enhanced response in females when compared to their male counterparts.1,3 Through ovariectomy in mice Mortola et al.3 eliminated gonadal hormones as a determinant of the observed differences.
Females had a higher number of chief cells compared to their male counterparts in all age groups. These cells were light staining with a euchromatic nucleus reminiscent of highly active protein synthesizing cells. The chief cells are responsible for chemoreception and chemoresponse.21 These cells produce a wide range of neurotransmitters including dopamine, noradrenaline and substance P.22 Accordingly, the higher number and evidence of activity may be responsible for the heightened activity of HCB in females. Pertinent to this suggestion are observations that chemoreceptor hypofunction leads to decreased peripheral drive for ventilation.23 The transmitters released by the chief cells trigger a receptor potential and hence action potential on the adjoining afferent nerve.22 Indeed reduction in neuronal like cells causes loss of sensory capabilities in transmission of chemoreceptor signals.24
In both males and females, aging was associated with reduction in cellular, vascular and innervation density and increase in stroma. This was consistent with other reports.14,23 In females, however, the attenuation was less severe in the sense that cellular and vascular density was comparatively better preserved. This is consistent with preservation of CB function which occurs in aged females.25‒28 The preservation of function albeit with fewer cells is probably related to the suggestion that only a small number of type I cells in the CB is sufficient to sustain functional activity.25,29‒31
Sex histomorphological differences in the human carotid body characterized by higher population of chief cells, density of vascularity, innervation and blunted age related morphological attenuation in females may underpin differences in respiratory function and disease predisposition.
We are grateful to Margaret Irungu, Judy Machira, Jacob Gimongo, Acleus Murunga for technical support and Antonina Odock- Opiko for typing and editing the manuscript.
The author declares no conflict of interest.

©2018 Muthoka, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.