MOJ
eISSN: 2471-139X


Mini Review Volume 3 Issue 5
Department of Veterinary Medicine, University of Abuja, Nigeria
Correspondence: Itopa E Ajayi, Department of Veterinary Medicine, University of Abuja, Nigeria
Received: April 13, 2017 | Published: May 10, 2017
Citation: Ajayi IE. Neuro anatomical and physiological considerations for the role of the periaqueductal gray in expressing emotions. MOJ Anat Physiol. 2017;3(5):153–155. DOI: 10.15406/mojap.2017.03.00107
The midbrain periaqueductal gray is a crucial interphase between the forebrain and nuclei in the brainstem that directly control basic physiologic functions. Recently, the role of the midbrain periaqueductal gray has been evolving as it is found to also regulate the expression of emotions. However, literature around this brain area is meager. The current article sheds light on the known anatomy and physiology of the periaquductal gray. In the context of whole-brain functioning, gaps in the literature are highlighted, which could be investigated to enhance the current understanding of this brain structure.
Keywords: periaqueductal gray neurons, firing pattern, vocalization, freezing, tachypnea
PAG, periaqueductal gray; dmPAG, dorsomedial; dlPAG, dorsolateral; lPAG, lateral; vlPAG, ventrolateral; EMG, electromyograms
The midbrain periaqueductal gray (PAG) is made up of a heterogeneous mix of cells longitudinally organized around the cerebral aqueduct. The first evidence of specific neuronal populations in the area was produced by microinjections of glutamic acid, an excitatory amino acid, in the cats brain.1 A closer examination of the region revealed that the cells therein were made up of three major varieties: fusiform, multipolar and triangular-shaped neurons.2,3 This different cell types did not form any distinct gross subdivisions of the PAG even though particular clusters were predominant in separate areas. However, studies based on functional segregation of somatic and autonomic output identified cellular organizations in longitudinally distinct columns relative to the cerebral aqueduct. The columns include dorsomedial (dmPAG), dorsolateral (dlPAG), lateral (lPAG) and ventrolateral (vlPAG) columns.4‒6 Anatomical tracing studies7‒9 further buttressed this columnar differentiation by revealing projections from neurons in the dmPAG, lPAG and vlPAG to the ventromedial and ventrolateral medulla, leaving a wedge-shaped dlPAG column devoid of medullary projections.
Neuronal groups in the PAG columns form important components of the emotional motor system10 as each group exhibits specific innate survival behaviors and adaptive physiological changes. For instance, in cats, chemical stimulation of the lPAG elicits a host of defensive behavioral reactions including vocalization, pupillary dilatation, piloerection11 and a flight reaction12 while stimulation of the vlPAG elicits freezing or immobility.12 In rats, microinjection of kainic acid in the lPAG and vlPAG produces forward avoidance13 and a quiescent behavior,14 respectively. These emotional behaviors are often accompanied by autonomic activities such as specific adaptive breathing patterns, changes in blood pressure 4,12,15‒17 and micturition,18,19 all of which synchronously produce coordinated responses to environmental and emotional challenges.
Gaps in knowledge of the physiological properties of PAG neurons: future directions
While so much attention has been paid to the integrated somatic and autonomic functions influenced by directly exciting specific PAG neurons, less attention has been given to the activity pattern expressed by the neurons as they coordinate these changes in vivo. Although the firing patterns of single neurons in the PAG in response to micturition20 in cats, vocalization21‒23 in monkeys and nociception24 in rats have been demonstrated, neuronal firing pattern during a host of other activities regulated by the PAG remain unknown. Regarding micturition and vocalization, available studies20,21,23 have restricted interest to only neurons related to the specific motor pattern (micturition and vocalization). Thus, the activity patterns expressed by PAG neurons at rest remains unknown. Furthermore, in the works of Dusterhoft et al.,23 the activity pattern of certain PAG neurons were illustrated with specific descriptions of periods of neuronal activity including how activity incremented or stayed constant in correlation to vocalization. However, the details of firing pattern such as their rhythm was not mentioned. Kirzinger & Jurgens22 demonstrated the details of the firing pattern of PAG neurons following chemical and electrical stimulation of vocalization. In instances of chemical stimulation-evoked vocalization, even though recorded cells had pattern-correlated activity with vocalization, stimulation and recording occurred at different locations.
A primary emphasis in the evolving field of neuroscience should therefore be understanding the activity patterns of PAG neurons both at rest, and during specific somatic and autonomic activities. With the availability of adequate data, computational models can also be deduced and used for further behavioral exploration. Direct chemical stimulation of PAG neurons at previously identified sites need to be reproduced to validate, with precision, localized circuits capable of producing tachypnea, vocalization and freezing behaviors. The activity pattern of such neurons where then observed. In each case, PAG neurons firing patterns were correlated with electromyograms (EMG) from muscles responsible for producing the motor output.
The PAG and the selection of integrated responses
The involvement of PAG neurons in the emotional motor system, particularly in defensive behaviors4 and nociception24 has been extensively demonstrated. The expression of such complex behaviors is usually accompanied by corresponding motor and autonomic changes in respiration, blood pressure, pupillary dilatation, vocalization and micturition.25,26 The integration of all these functions suggests that activities of very discrete regions differ and may be responsible each for a different function. However, with synchronous convergence of individual activity general systems level settings are achieved, and consequently homeostasis.
In conclusion, the PAG is not involved in cognition and emotional perception or processing but it is a likely pathway maybe through which the forebrain particularly the limbic system regulates autonomic function and it is believed to be essential in integrating of various responses, such as respiration (Figure 1), vocalization, cardiovascular function and micturition for the expression of emotions during a survival challenge.27,28 However, the anatomic organization of inputs into the PAG, the synaptic mechanisms driving such inputs and the manner in which the inputs shape the output of the PAG are also unknown. This knowledge will be critical in making inferences about possible pathways and mechanisms that support the functioning of the midbrain periaqueductal gray.
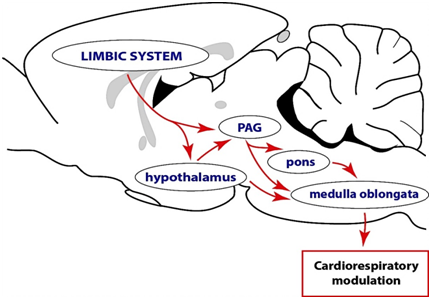
Figure 1 A conceptual illustration of the complex descending functional network through leading to modulation of motor and autonomic outputs. This proposal is in consonance with the Papez circuit, which argues that sensory messages concerning emotional stimuli first arrive at the thalamus. From the thalamus information is transmitted to the limbic system for cognition. The limbic system, in turn, projects to the hypothalamus and periaqueductal gray (PAG), both of which are capable of interacting with the various brainstem nuclei directly involved in motor and autonomic control. These modulatory pathways may function individually or in complements to achieve the expression of behaviors. Note that cardio respiratory out flow alterations are not the only indications of changes in the autonomic nervous system. Other indicators of changes in the autonomic nervous system include thermal and endocrine parameters.
None.
Author declares that there is no conflict of interest.

©2017 Ajayi. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.