MOJ
eISSN: 2471-139X


Research Article Volume 1 Issue 4
Departamento de Biodiversidad y Biologia Experimental, Universidad de Buenos Aires, Argentina
Correspondence: Andrea G Pozzi, Departamento de Biodiversidad y Biologia Experimental, Universidad de Buenos Aires, Argentina , Tel 54-11-45763384
Received: October 26, 2015 | Published: November 24, 2015
Citation: Raices M, Jungblut LD, Paz DA, et al. Cytotoxic effect of 5-bromo-2-deoxyuridine on olfactory epithelium. MOJ Anat Physiol. 2015;1(4):110–116. DOI: 10.15406/mojap.2015.01.00023
In vertebrates the most notable feature of the olfactory epithelium (OE) is its ability to continually replace cell types, including mature sensory neurons. In amphibians, the neuronal turnover is observed in adult animals and during larval development also. Today, one of the most used techniques for studies of cell proliferation and differentiation is the 5-bromo-2-deoxyuridine (BrdU) uptake, a synthetic analogue of thymidine that is incorporated into cells during DNA replication. Despite this, and because of its toxicity, some authors have questioned its use for the above mentioned purposes. This work analyses BrdU incorporation technique for cell proliferation and differentiation studies within the OE of amphibian larvae. The methodology used has been validated in Xenopus laevis larvae for studies related to inner ear formation. However, we observe a large number of adverse effects on cellular components of the OE, such as an increase in apoptosis, alterations in the expression pattern of different cell markers, as well as defects in the foraging behavior in animals that were exposed to BrdU. We conclude that even though BrdU can be successfully used as a proliferation cellular marker in the OE, It should be very careful when BrdU is used for studying cell lineage.
Keywords: amphibian, olfactory system, brdu, neurotoxicity, apoptosis
BrdU, 5-bromo-2'-deoxyuridine, OE, olfactory epithelium, aCasp-3, caspase 3 activated, OMP, olfactory marker protein, NCAM, neural cell adhesion molecule
The 5-bromo-2'-deoxyuridine (BrdU), is a thymidine analogue that is incorporated into the DNA of proliferating cells during the S-phase of the cell cycle.1 Today it is widely accepted as an indicator of cell proliferation.2 This compound is extensively used for the study of mitosis and cell lineage, both in vitro and in vivo. The use of BrdU can be easily combined with several phenotypic markers such as those used for neuronal or glial cells.3,4
BrdU is usually administered through intracerebroventricular, intraperitoneal, intravenous injections or orally via drinking water to study neurogenesis in the adult rat brain.5 In 2010, Kondo4 and co-workers injected BrdU at different stages of postnatal mice and observed chronological changes produced in BrdU-labeled cells in the OE, concluding that the use of BrdU was functional to demonstrate that it takes around 7 days for newly divided basal cells (BCs) to differentiate into mature olfactory receptor neurons (ORNs).4 In Xenopus laevis, it was shown that intraperitoneal injections of BrdU in juvenile and adult frogs allowed monitoring cellular origin, migratory behavior and cell differentiation patterns in the brain.3 Even though BrdU labeling is currently the most used method for studying adult neurogenesis, its use is also not without pitfalls and limitations.6‒8 Although extensively used in different biological systems, BrdU induces many side effects that may have consequences in the study and analysis of adult neurogenesis, as it may lead to false results and misinterpretation if appropriate controls are not performed. It was reported that BrdU can act as a mutagen and cell cycle delayer, alter DNA stability, cause disturbances in the plasmatic membrane and even trigger cell death pathways.9‒11 In pregnant rats, BrdU administration affects the litter size, body weight and mortality rate of the offspring. Moreover, it also produces decrease in cell proliferation, migration and patterning of the cerebellum in the adult progenies.5 Even if most studies in olfactory neurogenesis are performed on rodent, amphibian larvae represent an excellent model as well. They are a suitable option for many reasons. First, BrdU can be added directly into the rearing water tadpoles inhabit.12 Moreover, these animals have a completely functional olfactory system containing all the representative cell types of a mature system, which in addition possesses embryonic characteristics, so that the mechanisms of cell proliferation and differentiation are particularly active and provide a good opportunity to study regenerative processes.13 The aim of the present work is to examine the effect of BrdU incorporation on the cells phenotype in the OE.
Animals
Rhinella arenarumtad poles obtained by in vitro fertilization according to Pozzi et al.14 were maintained in dechlorinated tap water, with constant photoperiod and temperature (12:12 h, 22°C). Tadpoles ranging from stages 35-37 according to Gosner15 were used. All experiments were performed in accordance with the principles of laboratory animal care of the Institutional Care and Use Committee of the Facultad de Ciencias Exactas y Naturales, UBA Res CD: 140/00, and the principles of NIH (publication 8523, revised 1985).
BrdU treatment
Tadpoles (n=5 per treatment) were treated by immersion in phosphate buffered saline (PBS) with 0, 1, or 10mM BrdU (SigmaB5002, St. Louis, MO) during 45min at room temperature (22-24°C) as described by Quick and Serrano.12 Then, they were maintained in dechlorinated tap water for different times (1, 3, 72h and 30days) prior to euthanasia; and processed by different techniques detailed below.
Tissue preparation and histology
Tadpoles were anesthetized by immersion in 0.1% MS222 and fixed in Bouin’s solution for 24h at 4ºC. Then they were dehydrated, and embedded in Histoplast (Biopack, Argentina). Serial horizontal sections were cut at 7 µm and mounted onto HiFix glass slides (HF-5001; InProt, TNT, Buenos Aires, Argentina) subjected to immunohistochemistry or stained with alcian blue (pH=3.5) or hematoxylin for histological studies.
Immuno histochemistry
General procedures for immunohistochemistry were performed as in our previous report.16 After being treated with 5% hydrogen peroxide (H2O2) solution, sections (n=5 per treatment) were blocked by preincubation with a 5% solution of nonfat dry milk and incubated overnight at 4ºC with primary antibodies: rabbit anti-Caspase 3 activated (aCasp-3)1/30 (Cell Signaling, Cat. 9661) or mouse anti-Cytokeratin II 1/300 (Developmental Studies Hybridoma Bank, University of Iowa). Samples were then treated with the appropriate biotinylated antibody, anti-mouse (1/300) or anti-rabbit (1/200) (Vector Laboratories, Burlingame, United Kingdom), followed by avidin–horseradish peroxidase–biotin complex (1/500) (Vectastain Elite ABC kit; Vector Lab, Burlingame, CA). The reaction was visualized by exposure to 3, 3´-diaminobenzidine tetrahydrochloride (DAB) staining kit (Dako, Carpinteria, CA). For aCasp-3 immunodetection, sections were incubated 10 min in 10mM sodium citrate buffer, pH 6.0 at sub-boiling temperature before H2O2 incubation.
Double immunofluorescence
For BrdU-OMP and BrdU-aCasp3 double detection, after being blocked with milk, sections (n=5 per treatment) were incubated overnight at 4ºC with primary antibody mouse anti-BrdU 1/100 (Amersham, GE Healthcare Kit, code RPN202), according to the manufacturer's protocol. Then sections were treated with anti-mouse rhodamine 1/100 (Millipore Corporation, MA) and subsequently with rabbit anti-OMP (olfactory marker protein) 1/800 (Sigma, St Louis, MO Cat. O7889), mouse anti-a Casp3 or goat anti Gap-43 1/50 (Santa Cruz Biotechnology, Cat. Sc-7458), followed by the appropriate biotinylated antibody and streptavidine Alexa 488. Images were analyzed by confocal laser microscopy (Olympus FV-30 attached to a microscope Olympus Bx-61). For BrdU-NCAM double detection, BrdU was visualized by DAB reaction. Then sections were treated with mouse anti-NCAM 1/50 (Dr U. Rutishauser, Developmental Studies Hybridoma Bank, University of Iowa) overnight at 4ºC followed by an anti-rabbit biotinylated and streptavidine Alexa 488 (Invitrogen, Molecular Probes Inc, OR). BrdU was analyzed by light microscopy and NCAM was analyzed by confocal laser microscopy.
Immunohisto chemistry quantification
Slides containing comparable sections of OE from each animal were selected, coded so that cell counts results were obtained in blind condition. A line (150µm of length) was drawn across each image at the level of the olfactory receptor neurons in the medial region of the OE. The number of OMP-positive (OMP+) or aCasp-3 cells in each selected area (3 fields per animal) was observed and counted in at different treatment times, including the respectively controls (without BrdU treatment, n=5). Because the diameter of anuran olfactory cells are approximately of 10μm and serial sections were cut at 7µm, we counted positive cells leaving one section between each, so there is low probability that labeled cell would be counted twice, causing an overestimation of proliferating cells.
Immunoblotting analysis
Total proteins were extracted from pooled fourteen OE resulting from 0, 1 and 10mM BrdU treatments (n=3). Tissue was homogenized in Tris-buffer (100mM NaCl, 10mM Tris, pH 7.4, 1mM, EDTA, 0.5% NP40, 1% Triton, 1mM PMSF) with 1X protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA and centrifuged at 8000xg for 10min at 4°C. Protein extracts were separated on 15% SDS-PAGE and transferred to PVDF membranes (Amersham Biosciences, Arlington Heights, IL). After blocking for 2h with 5% solution of non-fat dry milk, membranes were incubated overnight with primary antiserum rabbit anti-OMP 1/3000 in TTBS, then with biotinylated secondary anti-rabbit, and subsequently with horseradish peroxidase conjugated with streptavidin (Zymed Laboratories, San Francisco, Calif.). The reaction was visualized by exposure to DAB staining kit. This protocol was repeated 3 times per treatment.
TUNEL technique
Apoptotic cells were detected using a commercial kit (ApogTag, Chemicon, Millipore, Billerica, MA). Briefly, tissue sections were deparaffined, rehydrated and treated with Proteinase K (Dako) 20μg/ml, 15min at room temperature, washed in PBS and then incubated with Formaldehyde 4%, washed again in balance buffer. Immediately, they incubated in the reaction mixture containing the TdT enzyme for 60min at 37ºC. Finally, they were incubated with anti-digoxigenin-peroxidase antibody and then with DAB substrate. Images of the sections were captured by an optical microscope.
Behavioral experiments
A rectangular glass aquarium (28 x 14x 12cm3) served as the choice tank. A central line perpendicular to the long axis was drawn at the bottom of the tank dividing it into two equal zones, namely zones A and B (Supplementary Figure 1). The filtered water obtained from boiled spinach (200g in 200ml of water) was used as chemical food stimulus. Before each trial the choice tank was carefully cleaned and dechlorinated tap water was filled to 1L. Tadpoles treated with 1 and 10mM BrdU, 72h post-treatment(n=8 for each treatment) were used as experimental subjects. The animals were individually tested and starved for 24h prior to each trial. After 10-15min of acclimatization in the choice tank 400μl of the food stimulus was slowly injected in a fixed point in the zones A or B (randomized between trials). Simultaneously with the food stimulus, 400μl of water (control) was discharged at the fixed point of the opposite side. Each trial was videotaped for 4 minutes with a digital camera suspended above the experimental arena. From the videos obtained we recorded the time performing foraging behavior in the area where the stimulus was discharged.
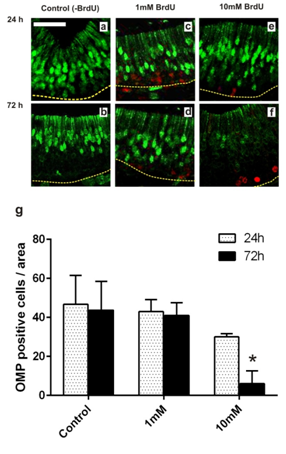
Figure 1 Effects of BrdU incorporation on the expression of Olfactory Marker Protein (OMP), a specific neuronal protein. (A-F) Microphotographs of homologous regions of olfactory epithelium of R. Arenarum tadpole (G36-37) at different post-treatment times (24 and 7h) with or without BrdU (1 and 10mM). (A, B) control animals; (C, D) 1mM BrdU; (E, F) 10mM BrdU. Double-immunostaining for BrdU (red) and OMP (green). Bar: 50mm. Dotted line indicates the basal lamina. (G) Quantification of OMP-positive cells as number of OMP-positive cells per area of equal length of the epithelium analyzed (n=3). *(P<0.05) vs. control group.
The results were presented as mean ± standard error (SEM). For quantification of apoptotic or proliferating cells and behavioral experiments, analysis was carried out using analysis of variance (ANOVA) and for semi-quantification of OMP by WB ANOVA in blocks was used. The homogeneity of variances within groups was verified with Bartlett´s test. When ANOVA demonstrated significant differences, a multiple comparison using the Tukey test was made. In all cases, results were considered significantly different when p<0.05. The statistical analyses were performed using Info stat software.
In order to establish the BrdU as proliferation tracer and subsequent study of cell fate in the olfactory epithelium, we treated tadpoles following the same protocol and conditions proposed by Quick and Serrano for the study of cell proliferation in X. laevis larvae.12 We assessed whether the incorporation of BrdU alters the cellular characteristics in the OE, studying changes in the expression of different olfactory cell markers. First, we examined the effect of BrdU on ORNs using OMP, a specific marker for mature olfactory neurons.17 Clear immunostaining of ORNs was observed with the antisera against OMP in the OE of stages 35-37 tadpoles. The antiserum to OMP stained the bipolar neurons whose cell bodies were located in the middle third of the OE and stained the luminal terminations of neurons (Figure 1A)( Figure 1B). We confirmed that the OMP distribution in non-treated animals does not change independently of the duration of the trial and/or larval stage reached during the study. Treatment with 1mM BrdU not caused a decline in the OMP-positive cells after 72h post-treatment (Figure 1C) (Figure 1D) (Figure 1G). Whereas, treatment with 10mM BrdU significantly reduced the number of OMP-positive cells as a function of the post-treatment time (Figure 1e). Histological analysis shows that substantial number of the olfactory cells is still maintained at 72hours after the BrdU administration (Supplementary Figure 2). This data could support the idea in which the decrease of the OMP-positive cells is caused by a down-regulation in expression of olfactory cells. Western blot analysis supported that 72h after 10mM BrdU treatment reduced in 60% the OMP levels (Figure 2). Moreover, neither OMP nor BrdU immunostaining were detectable after 72h post-treatment with 10mM BrdU (data not shown). Behavioral experiments showed that animals treated with 10mM significantly their normal foraging behavior in the presence of chemical food cues (Figure 3). This foraging behavior was similar to control when 1mM BrdU was administered. The alteration in foraging behavior could be explained not only by the loss of ORNs but also by a physiological alteration in those cells responsible for this olfactory function. Furthermore, we analyzed the effect of BrdU on the apoptosis evaluating an apoptotic marker, the activation of Caspase-3 (aCasp-3). When animals were treated with 1mM BrdU, the increase in the number of apoptotic cells was observed only after 24hours post-treatment. The administration of 10mM BrdU caused a significant increase in the number of immunoreactive aCasp-3 cells at 3h post BrdU-treatment. In all cases, the soma of apoptotic cells was observed in the ORN zone of the OE (Figure 4). Similar results were confirmed by TUNEL assay (Figure 5). Curiously, BrdU immunostaining never co-localized with aCasp-3 at any time analyzed (Figure 4G). Unfortunately, we did not co-localize BrdU with aCasp3 at any time or concentration tested. Perhaps, BrdU is exerting its effects on preexisting cells rather than on the proliferating cells. To evaluate if the negative effects were restricted to ORNs, we analyzed the effects of BrdU on the sustentacular cells (SCs), which provide the structural and functional support to the OE. The analysis of Cytokeratin II, a specific marker of SCs shows that BrdU treatment alters its pattern of distribution.18 In animals treated with 10mM BrdU, Cytokeratin II immunostaining disappears completely in cytoplasmatic projections and maintains only a weak stain in the apical region of the cell bodies (Figure 6A) (Figure 6C). Instead, treatment with 1mM BrdU showed differences only in cytoplasmic projections of SCs in the mid-basal area of the OE. This suggests that BrdU not only affects the ORNs but also causes structural changes in SCs altering their cytoskeletal organization. In addition, we analyzed the distribution of acid mucopolysaccharides vesicles. These glycoproteins contribute with the mucus formation on the OE surface and Alcian Blue staining revealed this typical secretion.19 At short times, BrdU significantly increased the vesicles content, appearing compacted in the apical zone of SCs (Figure 6D‒ Figure 6F).
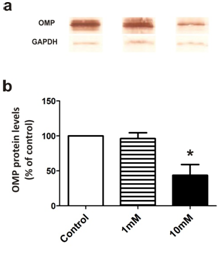
Figure 2 OMP protein level by western blot after BrdU treatment.
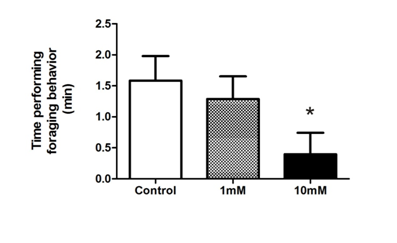
Figure 3 Effect of BrdU on foraging behavior. Animals were tested after 1 and 10mM or without (control) BrdU 72h post-treatment (n=8). The analysis included the time (min) performing foraging behavior (mean±SE) in the area where the stimulus was discharged. *(P<0.05) vs. control group..
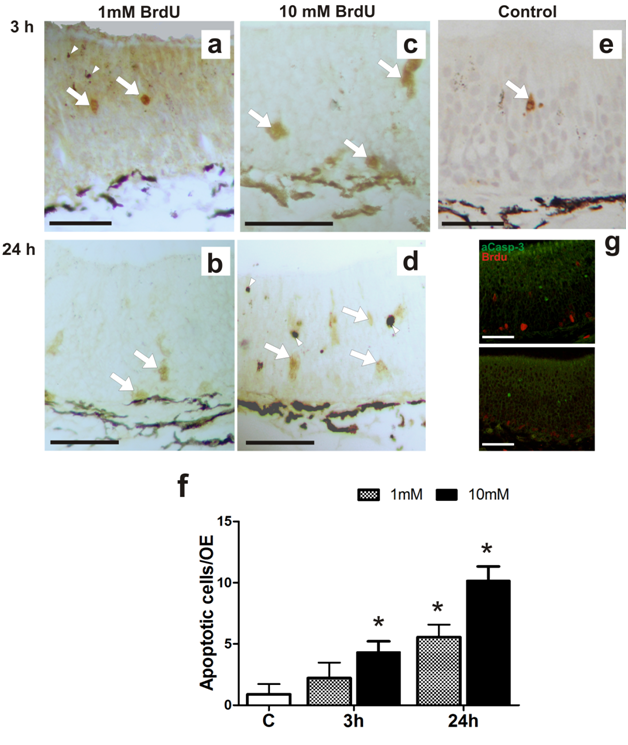
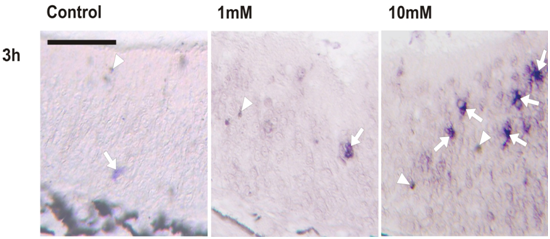
Figure 4 Effect of BrdU on caspase-3 activity. Effects of BrdU treatment on caspase-3 activated (aCasp-3) (n=3).
(A-E) Microphotographs of homologous regions of olfactory epithelium of R. arenarum tadpole (G36-37) at different post-treatment times (3 and 24h) with or without (control) BrdU (1 and 10mM). (A, B) 1mM BrdU; (C, D) 10mM BrdU.
(E) control animal. Bar: 50μm. Arrows: apoptotic cells, immunoreactivity for aCasp-3. Arrowhead: pigment
(F) Graphic representation of total number of a Casp-3 cells in the olfactory epithelium in control and 3h or 24h post-treated tadpoles (n = 3). *(P<0.05) vs. control group.
(G) Double-immunostaining for BrdU (red) and aCasp-3 (green) in larvae treated with 10mM BrdU after 3 or 24h post-treatment. Bar: 50μm.
Supplementary Figure 2 Microphotographs of homologous regions of olfactory epithelium of R. arenarum tadpole (G36-37) at 72h post-treatment with or without BrdU (1mM and 10mM), stained with Hematoxilyn. Dotted line indicates the basal lamina Bar: 50µm.
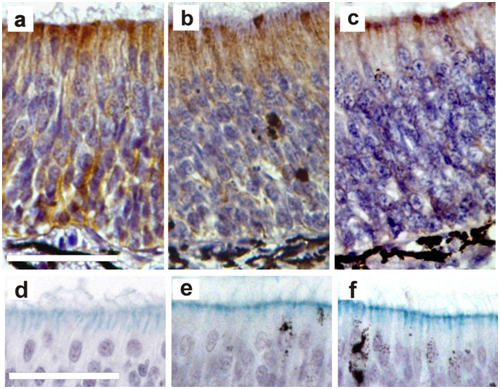
Figure 6 Effects of BrdU incorporation on sustentacular cells.
(A-C) Immunohistochemical detection of cytokeratin II (a specific marker for substentacular cells) in control (a) and 1mM (B) or 10mM
(C) BrdU treated animals (72h post-treatment): Bar: 50μm. (d-f) Histochemical detection of acid mucopolysaccharides with Alcian Blue pH=3.5 in control
(D) and 1mM
(E) or 10mM
(F) BrdU treated animals (3h post-treatment). Bar: 50μm.
In summary, these results demonstrate that while the general histological integrity of the OE in BrdU-treated animals seems not be changed, 10mM BrdU causes unwanted effects on both, ORNs and SCs in the OE. Treatment with 1mM BrdU provokes attenuated toxic effects that would not affect the functionality of the epithelium. Either 1mM or 10mM elicited changes in secretory vesicles of the SCs. Considering that low concentrations of BrdU did not alter the number of OMP-positive cells (Figure 1C) (Figure 1D), we analyzed whether the basal cells incorporating BrdU can begin a neuronal differentiation pathway. We analyzed the colocalization of BrdU with different neuronal markers: neural cell adhesion molecule (NCAM), a typical antigen that is expressed in differentiating neurons20 and Gap43 which is expressed in immature neurons (Figure 7). Cells that incorporated 1mM BrdU were located both at the basal and middle area of the EO. Independently of the time after treatment, none of them colocalized with NCAM immunoreactive cells (Figure 7C) (Figure 7D). However, Gap43 immunostaining colocalized with BrdU in the basal area of OE just at day 30 post-treatment (Figure 7b).

Figure 7 Effects of BrdU incorporation on neuronal differentiation.
(A,B) Double-immufluorescence for BrdU (red) and Gap43 (green), a specific in immature neurons.
(C,D) Double-immunostaining for BrdU (black) and neural cell adhesion molecule (NCAM) (green).
A typical antigen expressed in differentiating neurons. Both co-localizations were analyzed in tadpoles treated with 1mM BrdU after 3h or 30days post-treatment. Dotted line indicates the basal lamina. White arrows indicate colocalization sites. Bars: 50µm.
The use of BrdU exogenously administrated for proliferation, migration, cell differentiation and final fate in neural tissues studies, has broadly been used.2‒4 With regards to the methodologies used to analyze neurogenesis and cell lineage in the EO, BrdU incorporation is controversial and its toxicity should be considered when evaluating the results.21,22 The toxicity of BrdU was demonstrated in Rhesus monkeys (Macaca mulatta) embryos. Treatment with BrdU compromises the number of labeled cells and/or the number of survival cells.22 In our study, we show that BrdU incorporation in proliferative cells of the tadpole OE has a toxic effect in a dose-dependent manner. In previous works in our laboratory and others12,23 the amphibian larvae immersion in BrdU solution was used to evaluate cell proliferation, however, in these works the analysis was done at short time post-treatment and did not examine cell linage or neuronal differentiation. Based in our current results, it is feasible to use 10mM BrdU solution as a proliferation marker in the OE, but this analysis should be restricted to only a few hours post-treatment. After 72h post-treatment numerous alterations are observed:
These last alteration is probably a consequence of whole BrdU effects on the different OE components. Moreover, preliminary results confirmed these unfavorable effects of high doses of BrdU on olfactory neurons physiology. Golf gene expression, a specific-G protein involved in odorant signal transduction showed a reduction at 72h post BrdU-treatment. This reduction was observed using 10mM but not with 1mM BrdU (unpublished data). Based on the position of aCasp-3 in the OE, it is possible that BrdU induces death in basal cells but mainly on preexisting (mature) ORNs. However, this effect on apoptosis cannot be attributed only to the BrdU incorporation in proliferating cells but also should be considered the exogenous BrdU effects because it is possible that BrdU in aqueous solution could act as a harmful compound altering the OE both structurally and functionally. Some substances, which cause neurotoxic effects on ORNs,24,25 have been shown to alter the OE protein-signaling pathway. In zebrafish, copper toxicity,26 causes a negative effect on calcium channels, ion transport, and G protein-coupled olfactory receptors. This could be a possible explanation for ORNs death in the OE and the subsequent decrease in OMP labeling. Caldwell et al.27 have observed an increase in the number of apoptotic cells in cultures of rat neuronal progenitor cells after treatment with increasing BrdU concentrations. The neuronal population decays to 30% when compared to controls and the decrease is proportional to the BrdU concentration.27 We obtained a similar result, with 10mM BrdU detecting an increase in the number of apoptotic cells measured by aCasp-3 and TUNEL. No colocalization between BrdU and aCasp-3 positive cells was detected 3 and 24h after treatment (Figure 4G) and 72h after treatment no BrdU positive cells were detectable in any layer of the OE (Figure 1). A plausible explanation for the later is that after cellular division, the immunostaining was not detectable anymore due to a “dilution effect”. However, when we used 1mM, BrdU positive cells were still observed after 72h (Figure 7), so the dilution effect can be rejected. All these evidence is supporting the idea that these cells are dying and that is why the immunostaining was not detectable anymore.
Currently, other authors have shown that BrdU exposure of mice neural stem cells causes a decrease in DNA methylation. This demethylation produces a lost in stem cells marker’s expression and a change in the differentiation pathways to glial cells, which expresses GFAP, a specific astrocytes marker.10 This evidence partially correlates with our results: BrdU may have a negative effect on neuronal differentiation by preventing its maturation process. However, in our model, BrdU does not seem to increase glial cells; conversely, we observed a decrease in Cytokeratin II immunostaining, s glial cells marker. Strikingly, even if an increase in the number of apoptotic cells is observed, there is no change in the epithelium thickness between control and treated animals. The typical structure remains as a pseudo stratified epithelium with 7 or 8 cell layers from the base to the light. Based on the results of Schneider and d'Adda di Fagagna,10 wherein BrdU altered patterns of cell differentiation, we can suggest that BCs alter their neuronal differentiation pattern, with a normal histological appearance, which is probably not functional. This fact could account for the decrease in the efficiency of olfactory stimuli detection and be partially responsible for the decrease in the number of OMP-positive cells.
On the other hand, 10mM BrdU treatment provokes an alteration in Cytokeratin II expression. Among Cytokeratin functions, we can mention the preservation of the epithelial structure, mechanical protection and communication between cellular components of the OE.28 We conclude that changes in Cytokeratin II expression and acid mucopolysaccharides distribution can result in structural deficit and protective functions of the SC in animals exposed to BrdU. Our results also show that it is possible to reduce 10 times the dose of BrdU used since:
However, though small, we still observe an increase, in the number of apoptotic cells. For this reason when the purpose is not exclusively to assess cell proliferation one should be very careful because the intercalation of BrdU into DNA can alter the function and dynamics of the structure to which these cells belong. It is also remarkable that, despite the adverse effects that have been reported for BrdU, some cells survive its incorporation and although delayed in time, undergo a differentiation process. This suggests that some cells can avoid the adverse effects of BrdU. By which mechanisms this is achieved, may be an interesting matter to elucidate. Finally, we conclude that the results of BrdU cell labeling should be carefully assessed since crucial events such as the magnitude of cell division; pattern of cell migration and final differentiation could be altered by BrdU incorporation.
None.
Author declares that there is no conflict of interest.

©2015 Raices, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.