MOJ
eISSN: 2576-4519


Research Article Volume 6 Issue 1
1BVBGR-LR11ES31, Higher Institute of Biotechnology of Sidi Thabet (ISBST), University of Manouba, Tunisia
2Faculty of Sciences of Tunis, University of Tunis El Manar, Tunisia
3APVA-LR16ES20, National School of Engineers of Sfax (ENIS), University of Sfax, Tunisia
Correspondence: Mohamed Neifar, National School of Engineers of Sfax (ENIS), BP 1173, 3038 Sfax, Tunisia
Received: June 06, 2022 | Published: August 1, 2022
Citation: Sghaier I, Ouertani A, Ouertani R, et al. Eco-sustainable bioremediation of industrial azo-dye micropollutants by extremophilic plant growth promoting bacterium Halomonas desertis G11. MOJ App Bio Biomech. 2022;6(1):13-21. DOI: 10.15406/mojabb.2022.06.00161
Application of extremophilic plant growth promoting bacteria (PGPB) and their enzymes in bioremediation have been received increasing interest due to their eco-friendly nature and effectiveness for bio treatment of diverse industrial micro pollutants. In this work, the azo-dye decolorization potential of halophilic PGPB Halomonas desertis G11 was evaluated and optimized using central composite experimental design and response surface methodology. Interestingly, the increase of pH and NaCl concentration accelerated the dye decolorization. The model predicted a maximum removal of BEZACTIV blue S-2G dye (80%) at optimal operating conditions (dye concentration of 50 mg/L, inoculum size of 1.0%, pH of 8.2, NaCl of 5.0% and incubation time of 10 days). The experimental design model predictions are in good agreement with the experimental data, thereby providing the soundness of the developed model. The biodecolorization under pressures of high salinity and alkalinity seems to be correlated to azoreductase activity. The gene encoding FMN-dependent NADH azo-reductase from halophilic bacterium H. desertis G11 was identified and the structure and catalytic mechanism of dye decolorizing enzyme were elucidated. Results of this study provide evidence for the potential application of this azoreductase producing extremophilic bacterium as a novel candidate in the biological treatment of sediments and wastewaters contaminated by azo-dyes.
Keywords: Halomonas desertis,azo dye decolorization, azoreductase structure, experimental design, optimization
Synthetic dyes are extensively applied in textile, leather, tannery, paper and pulp, color photography, food, pharmaceutical, and cosmetic industries. About 700000 tons of synthetic dyes are annually produced in the world.1-3 They show considerable structural diversity with more than 3000 different varieties. Azo dyes are more cost effective to produce compared to other dyes.4 The synthetic dyes released into the industrial wastes were about 10 to 15%.5 Dye structures are generally resistant to light, temperature and chemical attacks rendering them highly recalcitrant. The release of these compounds into effluents, even at concentrations lower than 1mg/L, causes abnormal coloration of surface waters capturing the attention of both the public and the authorities and causing serious environmental and health hazards.2,5 Synthetic azo dyes have adverse health effects due to their carcinogenic and mutagenic toxicity,2 causing disorders such as nausea, hemorrhage, ulceration of the skin and mucous membranes, damage to the kidneys, reproductive system, liver, brain and central nervous system.5 The drawbacks caused by recalcitrant synthetic dyes have pushed the researchers and industrialists either to use natural dyes for textile due to their ecology, minimum impact on the environment and pollution6 or to develop green and efficient bio treatments before the textile wastewaters are released into the environment.7 The conventional physico-chemical methods of the treatment of dye containing wastewaters are not cost-effective and may result in the formation of hazardous by-products. Therefore, there is an urgent need in the development of robust and environmentally friendly approaches for the treatment of these wastewaters. The use of biocatalysts (microorganisms and their enzymatic systems) for the removal of synthetic dyes from industrial effluents offers considerable advantages. The bioprocess set-up and the running costs are relatively inexpensive; thus, dye biodegradation is a promising tool for the treatment of dye-containing wastewaters.3
Bacteria are frequently applied microorganisms for textile wastewater treatment since they are easy to grow aerobically or anaerobically under neutral to alkaline conditions. Halophilic and halotolerant bacteria can be considered as promising candidates for biodecolorization processes as they are able to grow easily at high concentrations of salts ranging from 3.5 to 20% NaCl.7,8 Moreover, some of them resist to other stresses such as heavy metals and toxic oxyanions which are so common in textile wastewaters. Several studies have been focused on halophilic bacteria and their abilities for decolorization of azo dyes.9,10 Bacterial azoreductases are reported as the mainly responsible enzymes for dye decolorization. Bacterial azoreductases are key players of xenobiotic metabolism. They are mainly involved in the cleavage of the azo linkages (-N=N-), resulting in azo dye biotransformation and bio detoxification. Although azoreductases reduced efficiently some azo dyes, certain azo dyes were not efficiently catalyzed. To establish biocatalyst-based system for textile azo dye degradation, it is advantageous to select bacterial halostable azoreductases with broad substrate specificities.11
Halomonas strains are Gram-negative rod-shaped bacteria isolated from diverse saline environments, such as ocean water, salterns, marine hydrothermal vents, hypersaline lakes and salted fermented food.12,13 They have numerous applications in various fields of industry due to their ability to produce commercially valuable metabolites and to degrade recalcitrant xenobiotic compounds such as aromatic hydrocarbons, pesticides and dyes.14 Some halophilic Halomonas strains have been recently isolated and tested for azo dyes decolorization and detoxification.9,10 However, only two azoreductase genes have been identified from these halophilic bacteria.9,15 Thus, the objective of this study is to achieve the extremophilic hydrocarbonoclastic PGPB Halomonas desertis G1112,16 which has been isolated from Chott El-Djerid salt-lake of Tunisian desert, for decolorization of textile reactive azo dyes under saline and alkaline conditions. The biodecolorization processes were studied and optimized using an experimental design and response surface methodology. The gene encoding azoreductase and enzyme structure were also identified.
The textile azo dyes Bezactiv Blue S-2G (λ max = 592nm), Bezactiv Blue S-Matrix (λ max = 600 nm), Bezaktiv Red S-Matrix 150 (λ max = 524 nm), Tubantin Brown GGL HC (λ max = 430 nm), Tubantin Blue BRR (λ max = 584 nm) and Tubantin Orange GGLN 200-FDS (λ max = 415 nm) were procured from a textile industry in Nabeul, Tunisia, and were of commercial quality. Absorbance maxima (λ max) of the dyes were measured in diluted dye aqueous solutions by using the UV-Vis scanning spectrophotometer (Shimadzu UV-1800 PC model Kyoto, Japan).
Bacterial strain and decolorization experiments
The hydrocarbonoclastic extremophilic plant growth promoting bacterium Halomonas desertis G11 was isolated from a salt-lake sediment.12,16 The decolorization experiments were conducted in 250 mL flasks containing 100 ml nutrient broth with peptone, sodium chloride, yeast extract and beef extract at 5, 5, 2 and 1g/L, respectively. The prepared media were supplemented with appropriate dye concentration. Thereafter, the flasks were inoculated with diluted overnight culture of H. desertis G11 (O.D. 600 nm ≈ 0.6) and incubated at 37°C under static condition. Abiotic controls and controls with dyes and without inocula were run under similar conditions. Dye decolorization (%) was calculated by measuring the decrease in the corresponding maximum absorbance of each dye in control and culture supernatants using an UV-Vis Spectrophotometer and expressed by the following the formula: Decolorization % = [(A0 − At)/A0] × 100, where A0 is the initial absorbance of the dye and At is the observed absorbance after time t of treatment .
Modelling and RSM optimization
To optimize Bezactiv Blue S-2G (BB S-2G) decolorization by H. desertis G11, a Central Composite Design (CCD) and Response Surface Methodology (RSM) were applied to study the decolorization reaction variables namely dye concentration, X1 (50-250 mg/L), inoculum size, X2 (1-5%), pH, X3 (6-9), salinity, X4 (0-5% of NaCl) and incubation time, X5 (4-10 days). The following equation was used to establish the CCD quadratic model:
Where Y is the response; Xi and Xj are independent factors; and b0, bj, bjj and bjk are intercept, linear, quadratic and interaction coefficients, respectively.
The data obtained from the CCD with regards to dye decolorization were subjected to analysis of variance (ANOVA) using Fisher’s F-test to check for errors and the significance of each variable.7,8 The effect of factors interaction on dye decolorization by H. desertis G11 was investigated by plotting 2D and 3D RSM contour plots of two independent factors while keeping the third factor at constant level. The generation and treatment of CCD data were performed using NemrodW software.17
Enzyme activity assays
Azoreductase activity assay was conducted as described by Tian et al.9 The activity of H. desertis azoreductase was estimated by measuring the reduction of methyl red at 430 nm. The reaction mixture (1000 µL) contained phosphate buffer (pH 8.0), 25µM azodye, 0.1 mM NADH, 0.5 µM FMN, and 100 µL azoreductase crude extract. The extinction coefficient of the dye is 23,360 M−1 cm−1. One unit of enzyme activity is defined as the amount of azoreductase needed for the reduction of 1 µmol methyl red per minute. The soluble proteins in enzymatic extract were quantified by the dye binding assay method of Bradford18 using bovine serum albumin (BSA) as a standard. Specific activity is defined as units of enzyme activity per mg of soluble proteins in the enzymatic extract.
Phylogenetic and structural analysis
The BLAST program at NCBI (http://www.ncbi.nlm.nih.gov/BLAST/) was used for azoreductase amino acid sequence homology searches. Multiple sequence alignment was performed by using Muscle Alignment Algorithm and Phylogenetic tree file was generated by One Click phylogeny analysis.19 The structure of azoreductase enzyme was predicted using Iterative Threading Assembly Refinement server (I-TASSER), the online structure prediction tool.20–22 The best model was selected according to their C score. A final analysis with CCP4MG molecular-graphics software was assessed to validate and to analyze the quality of the structures.24
Indiscriminate release of saline colored industrial wastewater into the environment has become today a serious ecological problem. Extremophiles are one of the most attractive biological tools for azo-dye degradation in the harsh condition of industrial textile effluents.6,25 Factors, like pH, salinity, and dye concentration, inoculum size and incubation time have a great effect on dye removal by microorganisms.7,8,26–28
A CCD and response surface methodology was applied in order to investigate the influence of experimental parameters (dye concentration (X1), inoculum size (X2), pH (X3), salinity (X4) and incubation time (X5) on the decolorization of Bezactiv blue S-2G by the extremophilic bacterium H. desertis G11 and to determine their optimal levels (Table 1).
|
N° Exp |
X1 |
X2 |
X3 |
X4 |
X5 |
Dye (mg/L) |
Inoculum size (%) |
pH |
NaCl (%) |
Time (days) |
Measured decolorization (%) |
Estimated decolorization (%) |
|
1 |
-1 |
-1 |
-1 |
-1 |
1 |
50 |
1 |
6 |
0 |
10 |
1.57 |
1.858 |
|
2 |
1 |
-1 |
-1 |
-1 |
-1 |
250 |
1 |
6 |
0 |
4 |
14.64 |
14.638 |
|
3 |
-1 |
1 |
-1 |
-1 |
-1 |
50 |
5 |
6 |
0 |
4 |
25.95 |
24.928 |
|
4 |
1 |
1 |
-1 |
-1 |
1 |
250 |
5 |
6 |
0 |
10 |
4.2 |
4.521 |
|
5 |
-1 |
-1 |
1 |
-1 |
-1 |
50 |
1 |
9 |
0 |
4 |
13.33 |
12.818 |
|
6 |
1 |
-1 |
1 |
-1 |
1 |
250 |
1 |
9 |
0 |
10 |
19.56 |
20.39 |
|
7 |
-1 |
1 |
1 |
-1 |
1 |
50 |
5 |
9 |
0 |
10 |
13.38 |
13.19 |
|
8 |
1 |
1 |
1 |
-1 |
-1 |
250 |
5 |
9 |
0 |
4 |
0 |
-0.479 |
|
9 |
-1 |
-1 |
-1 |
1 |
-1 |
50 |
1 |
6 |
5 |
4 |
5.59 |
4.924 |
|
10 |
1 |
-1 |
-1 |
1 |
1 |
250 |
1 |
6 |
5 |
10 |
36.7 |
37.377 |
|
11 |
-1 |
1 |
-1 |
1 |
1 |
50 |
5 |
6 |
5 |
10 |
24.61 |
24.267 |
|
12 |
1 |
1 |
-1 |
1 |
-1 |
250 |
5 |
6 |
5 |
4 |
21.98 |
21.347 |
|
13 |
-1 |
-1 |
1 |
1 |
1 |
50 |
1 |
9 |
5 |
10 |
75.11 |
75.276 |
|
14 |
1 |
-1 |
1 |
1 |
-1 |
250 |
1 |
9 |
5 |
4 |
0 |
-0.123 |
|
15 |
-1 |
1 |
1 |
1 |
-1 |
50 |
5 |
9 |
5 |
4 |
61.55 |
60.407 |
|
16 |
1 |
1 |
1 |
1 |
1 |
250 |
5 |
9 |
5 |
10 |
16.03 |
16.229 |
|
17 |
-1 |
0 |
0 |
0 |
0 |
50 |
3 |
7.5 |
2.5 |
7 |
38.55 |
41.972 |
|
18 |
1 |
0 |
0 |
0 |
0 |
250 |
3 |
7.5 |
2.5 |
7 |
29.79 |
29.001 |
|
19 |
0 |
-1 |
0 |
0 |
0 |
150 |
1 |
7.5 |
2.5 |
7 |
30.68 |
30.023 |
|
20 |
0 |
1 |
0 |
0 |
0 |
150 |
5 |
7.5 |
2.5 |
7 |
26.39 |
29.68 |
|
21 |
0 |
0 |
-1 |
0 |
0 |
150 |
3 |
6 |
2.5 |
7 |
11.89 |
13.271 |
|
22 |
0 |
0 |
1 |
0 |
0 |
150 |
3 |
9 |
2.5 |
7 |
20 |
21.252 |
|
23 |
0 |
0 |
0 |
-1 |
0 |
150 |
3 |
7.5 |
0 |
7 |
20.62 |
21.387 |
|
24 |
0 |
0 |
0 |
1 |
0 |
150 |
3 |
7.5 |
5 |
7 |
38 |
39.867 |
|
25 |
0 |
0 |
0 |
0 |
-1 |
150 |
3 |
7.5 |
2.5 |
4 |
54.64 |
59.221 |
|
26 |
0 |
0 |
0 |
0 |
1 |
150 |
3 |
7.5 |
2.5 |
10 |
68 |
66.052 |
|
27 |
0 |
0 |
0 |
0 |
0 |
150 |
3 |
7.5 |
2.5 |
7 |
44 |
38.785 |
|
28 |
0 |
0 |
0 |
0 |
0 |
150 |
3 |
7.5 |
2.5 |
7 |
37.13 |
38.785 |
|
29 |
0 |
0 |
0 |
0 |
0 |
150 |
3 |
7.5 |
2.5 |
7 |
45.76 |
38.785 |
Table 1 Experimental conditions of the CCD design in coded and natural variables and the corresponding experimental and estimated responses
The regression equation for azo-dye decolorization (Y) can be presented as:
where Y are the G2 strain decolorization response (%); Xj: system factors (correspond to various parameters influencing the decolorization of bezactiv blue) and b0, bj, bjk and bjj: significant model coefficients.
ANOVA of the CCD model showed high significance of the model regression and insignificant lack of fit value (Table 2). The regression equation obtained indicated the R-Squared (R2) and Adjusted R-Squared (R2adjust) values of 0.988 and 0.956 respectively, suggesting a good adjustment of the CCD model to the experimental data and highlighting that the model could explain 98.8% of the variability in the response. The linear effects of X1, X3, X4 and X5, the quadratic effects of X2, X3, X4 and X5 and the interaction effects between factors X12, X13, X14, X25, B34, X35 and X45 were found to be significant indicating that the model terms are limiting variables for BEZACTIV blue S-2G decolorization (Table 3). These data indicate clearly that Bezactiv blue decolorization significantly enhanced by decreasing dye concentration and increasing pH, NaCl and incubation time. The contour plots (2D) and response surface curves (3D) illustrating the interaction effect between pH and NaCl concentration on BB S-2G decolorization were shown in (Figure 1). As highlighted by the red arrows, both salinity and alkaline pH are favorable for decolorization efficiency. This is advantageous for textile wastewater treatment because one of the most important characteristics of these effluents is their high alkalinity and salinity. Tian et al.9 reported a halophilic bacterium Halomonas sp. strain GT, able to decolorize the azo dye Acid Brilliant Scarlet GR at 10% NaCl. Additionally, Guadie et al.10 isolated and evaluated a new Halomonas strain for his potential to decolorize various azo dyes at salt concentrations as high as 200 g L-1. These results suggest that the extremophilic bacterium H. desertsi G11 could be used for decolorization and treatment of saline textile effluents.
Source of variation |
Sum of squares |
Degree of freedom |
Mean square |
Ratio |
Significance |
Regression |
11051.4 |
20 |
552.57 |
31.6304 |
*** |
Residues |
139.75 |
8 |
17.47 |
||
Validation |
98.17 |
6 |
16.36 |
0.7868 |
NS |
Error |
41.59 |
2 |
20.79 |
||
Total |
11191.2 |
28 |
|
|
|
Table 2 Analysis of variance (Anova) of the quadratic model corresponding to Bezactiv blue S-2G decolorization by extremophilic H. desertis G11
(***): significant at the level 99.9%; NS: non-significant
Noun |
Coefficient |
F. Inflation |
Mean square |
t.exp. |
Significance% |
b0 |
38.785 |
|
1.375 |
28.21 |
*** |
b1 |
-6.486 |
1 |
0.985 |
-6.58 |
*** |
b2 |
-0.172 |
1 |
0.985 |
-0.17 |
86.00% |
b3 |
3.991 |
1 |
0.985 |
4.05 |
** |
b4 |
9.24 |
1 |
0.985 |
9.38 |
*** |
b5 |
3.416 |
1 |
0.985 |
3.47 |
** |
b11 |
-3.298 |
2.78 |
2.669 |
-1.24 |
25.10% |
b22 |
-8.933 |
2.78 |
2.669 |
-3.35 |
* |
b33 |
-21.523 |
2.78 |
2.669 |
-8.07 |
*** |
b44 |
-8.158 |
2.78 |
2.669 |
-3.06 |
* |
b55 |
23.852 |
2.78 |
2.669 |
8.94 |
*** |
b12 |
-3.661 |
1 |
1.045 |
-3.5 |
** |
b13 |
-9.224 |
1 |
1.045 |
-8.83 |
*** |
b23 |
-2.205 |
1 |
1.045 |
-2.11 |
6.60% |
b14 |
-4.77 |
1 |
1.045 |
-4.56 |
** |
b24 |
0.771 |
1 |
1.045 |
0.74 |
48.70% |
b34 |
3.994 |
1 |
1.045 |
3.82 |
** |
b15 |
1.976 |
1 |
1.045 |
1.89 |
9.30% |
b25 |
-9.415 |
1 |
1.045 |
-9.01 |
*** |
b35 |
3.143 |
1 |
1.045 |
3.01 |
* |
b45 |
4.909 |
1 |
1.045 |
4.7 |
** |
b55 |
23.852 |
2.78 |
2.669 |
8.94 |
*** |
Table 3 Estimated effects, regression coefficients, and corresponding t and P values for Bezactiv Blue S-2G decolorization by G11 strain in central composite design experiments
(***): significant at the level 99.9%; (**): significant at the level 99%; (*): significant at the level 95%.
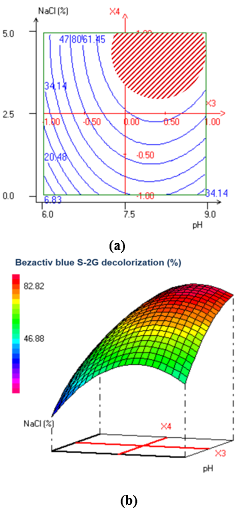
Figure 1 Contour plot (a) and response surface curve (b) showing interactive effect of NaCl concentration and pH on the decolorization of Bezactiv Blue S-2G by H. desertis G11 strain. Optimal zone is indicated by hatched-red region.
The optimum operating conditions of BB S-2G, carried out numerically by using NemrodW software are: dye concentration of 50 ppm, inoculum size of 1.0%, pH of 8.2, NaCl of 5.0% and incubation time of 10 days. The expected value of BB S-2G decolorization yield was Yop=80.11%. Additional experiment was carried out under the selected optimal decolorization conditions. It led to decolorization yield equal to 83.74%, which was in close agreement with the predicted response value. After testing the decolorization of BB S-2G, we expanded our analysis to a broader range of azo dyes. H. desertis G11 showed a remarkable ability to decolorize Bezactiv Blue S-Matrix 150 (89%), Tubantin Brown GGL HC (70%), Tubantin Blue BRR (56%), Bezaktiv Red S-Matrix (69%) and Tubantin Orange GGLN 200-FDS (65%) (Table 4). It was reported that decolorization variation depends on the structure and complexity of the dyes, particularly on the nature and position of the substituent in the aromatic rings.10,29 The G11 strain showed 76.4% of color removal of azo blue dye mixture at 10 days under saline conditions, suggesting that this bacterium could be used effectively for the treatment of real textile effluent as reported for other dye decolorizing halotolerant Halomonas strains (Table 4).
|
Strain |
Dye |
Concentration (mg. L-1) |
Percent (removal time) |
Medium NaCl content |
Reference |
|
Halomonas sp. GTW |
Reactive Brilliant Red K-2BP |
100 |
100% (24 h) |
150 g L−1 |
[34] |
|
Acid Red G |
50 |
100% (24 h) |
|||
|
Acid Red B |
50 |
100% (24 h) |
|||
|
Acid Scarlet GR |
50 |
90% (24 h) |
|||
|
Acid Black 10B |
50 |
90% (24 h) |
|||
|
Reactive Brilliant Red X-3B |
50 |
100% (24 h) |
|||
|
Halomonas sp. A3 |
Remazol Black B |
50 |
56% (96 h) |
50 g L−1 |
[35] |
|
Remazol Black N |
50 |
87% (96 h) |
|||
|
Sulphonyl Green BLE |
50 |
97% (96 h) |
|||
|
Sulphonyl Scarlet BNLE |
50 |
60% (96 h) |
|||
|
Sulphonyl Blue TLE |
50 |
85% (96 h) |
|||
|
Maxilon Blue |
50 |
46% (96 h) |
|||
|
Entrazol Blue IBC |
50 |
41% (96 h) |
|||
|
Mixture of above seven dyes |
50 |
100% (120 h) |
|||
|
Halomonas sp. Gb |
Remazol Black B |
50 |
64% (96 h) |
50 g L−1 |
[36] |
|
Remazol Black N |
50 |
82% (96 h) |
|||
|
Sulphonyl Green BLE |
50 |
95% (96 h) |
|||
|
Sulphonyl Scarlet BNLE |
50 |
74% (96 h) |
|||
|
Sulphonyl Blue TLE |
50 |
56% (96 h) |
|||
|
Maxilon Blue |
50 |
55% (96 h) |
|||
|
Entrazol Blue IBC |
50 |
32% (96 h) |
|||
|
Mixture of above seven dyes |
50 |
100% (120 h) |
|||
|
Halomonas sp. D2 |
Remazol Black B |
50 |
72% (96 h) |
50 g L−1 |
[37] |
|
Remazol Black N |
50 |
82% (96 h) |
|||
|
Sulphonyl Green BLE |
50 |
94% (96 h) |
|||
|
Sulphonyl Scarlet BNLE |
50 |
72% (96 h) |
|||
|
Sulphonyl Blue TLE |
50 |
56% (96 h) |
|||
|
Maxilon Blue |
50 |
37% (96 h) |
|||
|
Entrazol Blue IBC |
50 |
21% (96 h) |
|||
|
Mixture of above seven dyes |
50 |
100% (120 h) |
|||
|
Halomonas sp. strain A55 |
Reactive Red 184 |
50-150 |
≥98% (96h) |
0-200 g L−1 |
[10] |
|
Reactive Red 141 |
50-150 |
≥98% (96h) |
|||
|
Reactive Red 120 |
50-150 |
≥98% (96h) |
|||
|
Reactive Yellow 84 |
50-150 |
≥98% (96h) |
|||
|
Reactive Yellow 160 |
50-150 |
≥98% (96h) |
|||
|
Reactive Blue 198 |
50-150 |
≥98% (96h) |
|||
|
Vat Blue 43 |
50-150 |
≤48% (96h) |
|||
|
Real effluent |
- |
86% (96h) |
|||
|
Halomonas desertis G11 |
Bezactiv Blue S-2G |
50 |
80% (10d) |
50 g L−1 |
This work |
|
Bezactiv Blue S-Matrix |
50 |
89% (10d) |
|||
|
Tubantin Blue BRR |
50 |
56% (10d) |
|||
|
Bezaktiv Red S-Matrix 150 |
50 |
69% (10d) |
|||
|
Tubantin Brown GGL HC |
50 |
70% (10d) |
|||
|
Tubantin Orange GGLN 200-FDS |
50 |
65% (10d) |
|||
|
Mixture of above blue dyes |
50 |
76.4% (10d) |
Table 4 Halotolerant and halophilic Halomonas capable of azo-dye decolorization
UV–vis scan of samples before and after enzyme treatment suggested that the dye decolorization was accrued under degradation mechanism rather than inactive surface adsorption. To confirm the presence of azo dye degrading enzymes, the decolorized samples were collected under optimized conditions and compared with the control. Results showed that H. desertis G11 possesses azoreductase activities that play a significant role in the breakdown of the azo bond of the tested dyes. In fact, the presence of azo dyes has significantly stimulated G11 strain to induce azoreductase activities, which were about 2 to 3-folds greater than control (≈ 1.0 U per mg protein). Similar behavior was also reported by Guadie et al.10 who observed a significant increase in the activities of azoreductase in cells obtained after reactive red 184 decolorization indicates the involvement of this enzyme in the decolorization process.
Many azoreductases from different bacteria have been characterized, however very few of them especially flavin-dependent azoreductases are solved for the structure. Functionally, bacterial azoreductases can be classified as either flavin-independant or flavin-dependent azoreductases.9 The last ones can be classified as NADH-dependent, NADPH-dependent or dependent on both coenzymes. A BLAST search for azoreductase was performed in the genome of H. desertis strain G11. The phylogenetic tree analysis indicated that H. desertis azoreductase gene and its homologous azoreductases formed a unique branch among the NADH-dependent azoreductases (Figure 2). The requirement of NADH and NADPH for the azoreducing activity of H. desertis G11 was analyzed using the azo dye methyl red as a substrate.30 The decolorization rate was significantly faster with NADH, as an electron donor, than NADPH, indicating that H. desertis azoreductase prefers NADH as a source of reduction. This result agreed with that recorded by Guadie et al.10

Figure 2 Phylogeny of bacterial azoreductase proteins created using Phylogeny.fr. Phylogenic tree was generated using a multiple alignment of proteins (Muscle alignment) which have experimentally shown azoreductase activity. A specific colour is attributed to each azoreductases family: green for FMN-independent azoreductases; blue for FMN-dependent NADPH azoreductases and black for FMN-dependent NADH azoreductases. The nomenclature is the following: first the species and then the references of the protein sequences.
The predicted model for H. desertis G11 azoreductase indicates that the enzyme belongs to the flavodoxin like fold family with a molecular weight of 25.26 KDa and isoelectric point of 5.17. Structure analysis demonstrates that the enzyme is α/β fold structure and binds a Flavin mononucleotide (FMN) molecule at the active site. FMN binding plays an important role in fold stabilization.31 The α/β structure is composed of six α helices ( α1: His 24-Arg 40, α2: His 60-Phe67, α3: Ser 75-Ala 93, α4: Ala 110-Val 119, α5: Leu 171-Ile 182, and α6: Gly 199-Glu 225) and seven β strands (β1: Ile 4- Asp 8, β2: Leu 45-Asp 49, β3: Ile 96-Asp102, β4: Asp 127-Val 128, β5: Lys 140-Leu 141, β6: His 147- Arg 154, β7: Glu 193, Leu 187). Five parallel β strands formed a β sheet (β1, β2, β3, β6 and β7) and the two others are organized an a β turn β structure. The structure of azo-reductase showed the presence of five loops around the active site (loop 1: Pro 13- His24, loop 2: Thr 68-Ser 75, loop 3: Asp 129-Lys 140, loop 4: Arg 154-Ala 166, loop 5: Gly 198-Leu202) (Figure 3). The FMN binding site is composed of Arg 12, His 24, Ser 25, Tyr 104, Asn 105, Phe 106, Gly 156, Gln195, Gln196 (Figure 4). The textile azo dye reduction by the FMN-dependent NADH azoreductase from H. desertis G11 was performed through two-step mechanism, in each step two electrons were transferred from the enzyme cofactors to textile azo dye, which acts as a final electron acceptor, resulting in the production of colorless compounds. Eslami et al.15 reported the first study describing the sequence and activity of an azo-reducing enzyme from the halophilic bacterium Halomonas elongata. Although salinity has negative effect on enzyme activity, the azoreductase (AzoH) decolorize efficiently remazol black B, a representative of di-azo dyes, up to 15% (w/v). Halotolerant azoreductases cloned and characterized from other halophilic bacteria have been also reported to decolorize azo dye at high salinity levels.10,32–35 The stabilities of azoreductases at alkaline pH and higher salinity could make them more proficient for textile wastewater treatment under saline conditions.9
Although several bacteria have shown enormous potential for degradation of azo dyes, very few strains can be resistant to the dying effluent conditions in terms of pH and salinity. In this study, we shed light on the ability of the extremely alkali-halotolerant G11 strain to decolorize several textile azo dyes. The results obtained support the efficiency of CCD and RSM in elucidating the significance of different factors and predicting optimum conditions for Bezactiv Blue S-2G decolorization by halophilic Halomonas desertis G11. The significant increase in the G11 azoreductase activities obtained after azo dyes decolorization indicates the involvement of this enzyme in the biodegradation process. In the future, cloning, expression and purification of H. desertis NADH-dependent azoreductase will help us gain valuable insights into the characteristics of this enzyme of interest. Potential of the strain needs to be demonstrated for its application in the treatment and detoxification of real textile wastewater using appropriate bioreactors. Finally, the results of this study constitute a "proof of concept" and demonstrate that it is possible to develop a green, sustainable and robust biocatalyst very useful in designing a cross-flow continuous membrane enzyme bioreactor to effectively biotreat textile wastewater.
The authors acknowledge financial support from the European Union in the ambit of the project MADFORWATER (H2020, GA 688320), the PhosAgro/UNESCO/IUPAC partnership in the ambit of the Green Chemistry for Life project “Development of biofertilizers for a sustainable agriculture in Tunisia” and the Tunisian Ministry of Higher Education and Scientific Research in the ambit of the laboratory project LR11ES31.
The data used to support the findings of this study are available from the corresponding author upon request.
The authors declare that they have no conflict of interest.

©2022 Sghaier, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.