Journal of
eISSN: 2475-5540


Literature Review Volume 6 Issue 1
Regenerative Medicine, Faculty of Medicine, Arabian Gulf University, Bahrein
Correspondence: Haifa Abdullah, Regenerative Medicine, Faculty of Medicine, Arabian Gulf University, Bahrein
Received: November 19, 2019 | Published: February 5, 2020
Citation: Abdullah H, Alshammary S. Therapeutic application of stem cells in diabetes. J Stem Cell Res Ther. 2020;6(1):23?30. DOI: 10.15406/jsrt.2020.06.00137
Diabetes is a significant health problem in developing countries. The incidence of the disease is increased dramatically every year. The current medications are to control the levels of hyperglycemia and diabetic complications. Stem cells have the potential to differentiate into any specialized cells and help in improving the disease, especially in generating insulin-producing β-cells. This article summarized the advancement role of stem cell research for diabetic treatment.
Keywords: Diabetes, stem cell, regenerative medicine, cellular therapy, diabetes types, hyperglycemia
Diabetes is severe metabolic disorders characterized by high blood glucose levels (hyperglycemia).1 The prevalence of diabetes continues to increase worldwide, reaching to about 425 million people suffered from, and 629 million people would be expected to develop the disease by 2045. Type 1 (T1D) and two diabetes (T2D) are caused by an absolute or relative inadequate supply of functional insulin-producing β cells. As a result, patients with diabetes develop micro-and macro-vascular complications leading to morbidity and mortality.2 Due to the severe complications and huge costs associated with diabetes, researchers are investigating other advanced trials of treatment. Insulin or oral hypoglycemic agents are affected by diet and exercise, and islet transplantation. However, these types of therapies are limited because of its proper dosage and timing challenging as well as transplantation requiring not only rare donors but also immunosuppressant therapy to prevent rejection and overcome its complications. Hence, it is clear that these standard therapies for diabetes fail to copy the insulin discharge of healthy β cells, therefore not curative.3,4 Thus, the current therapies do not work like the healthy beta cells of the pancreas for secreting insulin; therefore, it is ineffective.
In this regard, stem cells offer promising treatment because they can replace damaged cells in the body, including the non-functional insulin-producing β cells of the pancreas.3
Biologically, stem cells can divide and differentiate into diverse cell types which are specialized for a specific action and renew themselves. For therapy, these cells are extracted, prepared, and administered into the body where they can repair the damaged tissues in the body, including the pancreas. Stem cells would be able to develop into normal insulin-secreting after activation with specific growth factors before injection in diabetic patients. Stem cells therapy would save the time and money spent on drugs-treatment.5 In this paper, we focused on the possibility of using therapeutic stem cell treatment for diabetes.
Aim
This study focused on the clinical application of stem cells in diabetic-treatment. For this purpose, clinical trials based on humans were studied following surveying 24 PubMed journals and Google scholar database from 1st January 2010 till 1st October 2018.
Search strategy and literature sources:
The analysis was performed with the following criteria, including stem cells, diabetes, insulin, clinical trials, and therapy.
Study selection:
Articles obtained were 72 restricted to title and abstract in order to manually detect any potentially relevant article. Exclusion criteria were as follows: (1) irrelevant articles, (2) studies on animals (3) meta-analysis studies are not critical because this study focuses on clinical trials, (4) studies on patients with morbid obesity, (5) effect of autologous stem cells on immune function in patients with diabetes because the main aim is to find a permanent cure and not finding the implications on immune system, (6) long-term effects of the implantation of stem cells for diabetes because of the possible adverse effects like cancer due to stem cell therapy, (7) assessment of drugs in stem cell transplantation in diabetes, (8) immune response after stem cell transplantation because the main concern is not to find immune response disturbances, (9) the prospect vaccination to prevent diabetes, (10) gene editing and risk of stem cell transplantation. The full text of studies meeting the criteria was retrieved as was the full-text of those that reported insufficient information to determine eligibility. Of 72 articles initially identified, 38 articles were included. Abstracts of these articles were evaluated for eligibility, and only 19 were selected for this research.
Data extraction
Twenty-five full-text studies were thoroughly analyzed, and the data were obtained as follows: (1) study type, (2) number of patients in the clinical trials, (3) stem cell definition, (4) delivery methods of stem cells, (5) therapy results and conclusions.
Stem cells and diabetes:
Definition:
Stem cells are primary unspecialized cells that can self-renew and differentiate into various specialized cell types. All organisms contain stem cells from the early stage of development at around 5-7 days after fertilization to the end of life. They play a critical role in the advancement, evolution, upkeep, and restoration of soft tissue and organs, for example, the brain, pancreas, bones, muscles, nerves, blood, and skin.6
Characteristics:
"Stem cells are mother cells having exclusive features by providing a favorable tool for tissue rebirth and body growth. These cells substitute impaired cells in each tissue of the body. Thus, during the previous years, stem cells have been utilized solely or in conjunction with other healing methods to treat a variety of ailments (Figure 1).7
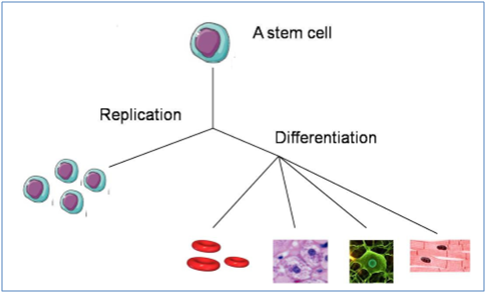
Figure 1 Features of stem cells: replication and diversity.8
Classification of stem cells:
Stem cells can be broadly classified based on their sources as below:
Embryonic stem cells:
These are derived from the embryos at approximately four to five days of a developmental stage during pregnancy. It is developed from inner cell mass of the blastocyst. Additionally, embryonic stem cells can be made outside the body using in vitro fertilization (IVF). The blastocyst has two layers, inner cell mass, and the outer layer. They are also called embryoblast and trophoblast, respectively. Embryonic stem cells formed after four to six days before implantation in the uterus. After that, the outer cell mass becomes a part of the placenta while the inner cell mass which is a group of a cell will differentiate to grow all the structure of an adult organism.6
Adult stem cells (ASC):
ASC are found through the organs like brain, blood, blood vessels, skeletal muscles, skin the liver, and the pancreas of the adult. These stem cells are essential to maintain and repair the tissue in which they are found. Adult stem cells are dormant for years and activated by disease or tissue injury.8
Induced pluripotent stem cells (IPS)
IPS are Adult cells are genetically reprogrammed in the laboratory to produce embryonic stem cell-like. IPS can differentiate into almost all specialized cell types of the body. Therefore, they can potentially generate new cells for any organ or tissue . IPS are used widely as disease-model and researches around the globe are hoping these cells can help to make a cure for many grave diseases. Not to mention because it is autologous therefore no less immunological reaction or rejection from transplanted tissueS that made by IPS.8
Diabetes:
Diabetes is a group of chronic metabolic diseases that are characterized by hyperglycemia along with disruption of carbohydrate, protein, and fat metabolism because of insufficient secretion of insulin, insulin resistance, or a combination of both.9
Diabetes types: 1 and 2 are the major types of this disease. Type 1 diabetes (T1D) accounts for 5-10% of all diabetic patients and develops in childhood and puberty. T1D is instigated by the obliteration of the insulin-producing β-cells in the pancreas due to auto-immune destruction, leading to an absolute insulin deficiency. Therefore, patients with T1D require injections of exogenous insulin in order to control their blood glucose and stay alive.9
Type 2 diabetes (T2D), is considered to be the most common type of diabetes, accounts for 90-95% of all diabetic cases and develops in adulthood. T2D is categorized by insulin resistance and comparative insulin deficiency. Patients with T2D may require insulin for control of hyperglycemia if this is not achieved with diet alone or with oral hypoglycemic agents.9
In T1D, insulin-producing β cells become non-functional due to auto-immune destruction of these cells leading to hyperglycemia. Traditional insulin therapy supports the control of blood glucose levels, but it has reported ineffectiveness in the long term. Another therapy that could be used is islet transplantation, but it is limited because of the availability of pancreatic cells, cell injection, use of immunosuppressive drugs, and other complications. Recently, stem cells are emerging as the best candidate for overcoming those limitations and effective therapy for T1D. Differentiating into mature beta cells is their unique ability in the existence of specialized signals. Differentiating from human embryonic stem cells into functional β cells was reported by several researchers in vivo. Moreover, adult stem cells such as hepatic, bone marrow, and umbilical cord blood stem cells have been discovered for their potential to generate insulin-producing β cells. For instance, it was found that human hepatic stem cells differentiated into insulin-producing β-like cells and used to overcome the condition of hyperglycemia. Thus, the application of stem cell to induce the regeneration of insulin-producing cells is successful, and its potential as T1D therapy is promising.10
In T2D, insulin resistance and a decrease in insulin production are the causes. Traditional therapy includes the use of oral hypoglycemic drugs and external insulin. However, the regular use of these drugs and insulin cannot cure this disease and prevent its complications. Although a promising therapeutic approach of transplantation of islet cells, this approach is not common due to the lack of donors, ethical conflict, and risk of immunogenicity. Regeneration and differentiation potential of stem cells make it an essential candidate for therapy. It was testified that embryonic, adult, and induced pluripotent stem cells are capable of differentiating into insulin-producing cells, causing an increase in insulin level in patients with T2D.10
Delivery methods of stem cells:
A few numbers of studies reported the route of administration of stem cells in patients with diabetes. Sood et al. injected stem cells via the peripheral intravenous route or targeted routes into the pancreaticoduodenal artery and splenic artery. The writers inferred that the intravenous progression was the least valid path for stem cell infusion. In any case, this deduction needs to be confirmed on a more significant sample examination as the previous analysis was carried out on the small specimen.11,12 A certain degree of success was attained when bone marrow stem cells were injected into the pancreas through the gastroduodenal artery in T2D.13 Mesples et al. injected bone marrow stem cells through the intrahepatic route in two T1DM patients resulted in increased C-peptide and hemoglobin A1c (HbA1c) levels.14 Another group transplanted stem cells through intravenous and intrapancreatic endovascular injection in 22 T2DM patients. This study showed that the signs of inflammation, glucose, and HbA1c levels were reduced and improvement of C-peptide levels.15 Nevertheless, the establishment of the exact pathway in stem cell delivery of diabetes still needs to be well established in larger sample size studies.
Applications of stem cells:
The therapeutic applications of stem cells are provided due to their high capacity of self-renewal and repairing the damaged tissues. Stem cell therapy has been proved to helps diabetic patient complications therapies might be either implanting stem cells into the body or redirecting them into growing and differentiating as a new tissue.8
Possible treatments by stem cells:
Ongoing researches on stem cell therapies revealed that stem cells could treat many diseases, including cancer, diabetes, Parkinson’s disease, cardiac failure, muscle damage, and many others (Figure 2). However, they have suggested more research to be conducted on stem cell therapeutics to understand cell behavior upon transplantation and mechanisms of stem cell interaction with the diseased/injured microenvironment before applying these therapies in the clinical setting.8
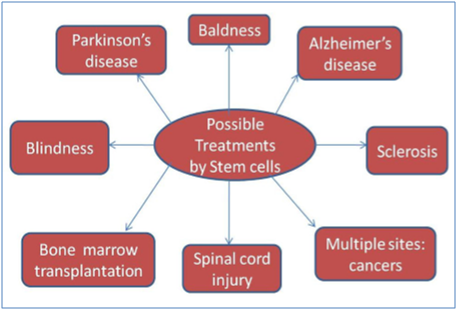
Figure 2 Possible treatment by stem cells.8
Stem cells for diabetes therapy:
Treatment of diabetes type 1 in clinical trials with humans:
2007 was a great year when, historically, Voltarelli and his colleagues evaluated the safety and viability of the stem cell transplantation in the first-ever clinical trial. They also assessed the effectiveness of the treatment. Stem cell transplantation using autologous nonmyeloablative hematopoietic stem cells was followed by the immunosuppressive therapy to stop the rejection response to the transplanted cells among patients. Insulin-producing cells degradations stop, and their function improves even further. This clinical trial included 15 patients who had T1D for six months. Patients who might develop complications. As the result of probable safety concerns in patients with a more significant threat of impediments, any patient with diabetic ketoacidosis was excluded from the trial. The follow up was done for the patients somewhere from 7 to 36 months, which indicated to irregularities in further monitoring. Out of 15, 14 patients turned into insulin-free conditions for inconstant periods during the follow up.3 The mean total C-peptide level of previous group of patients was quite high after six months of treatment. For 13 of the patients, HbA1c levels were sustained below 7%. Noticeably, these results support AHST as an effective treatment for T1D. However, one patient suffered pneumonia, and two patients developed late endocrine dysfunction.
Additionally, the majority of patients showed symptoms of neutropenia, nausea, vomiting, and alopecia due to the immunosuppressed treatments in addition to stem cells. About mortality, the rate was zero. However, this study showed limitations, including the absence of sample randomness and a control group, the shortness and inconsistent time of follow-up, and the limited small sample size.3
Two years later, a follow-up study was performed by the same research laboratory to deal with some limitations of their earlier experiments. The model size comprised 15 previous samples along with eight more patients, and the research was sustained for four more years yielding follow-up results. Patients with ketoacidosis were excluded as in the 2007 study for the treatment protocol. During the follow-up time, 20 patients reached insulin independence for 7 to 58 months, and among them, 12 patients sustained the insulin-free condition for 14 to 52 months. In all patients, C-peptide levels were considerably raised, and HbA1c levels were sustained under 7%. These results revealed the increased insulin production and β cell function in addition to maintained positive effects of the treatment after the prolonged follow-up period. The most frequent complication was oligospermia.3
Moreover, in two patients, bilateral nosocomial pneumonia was noted. This study added proof that stem cells are useful as a treatment for T1D, with the more significant part of patients attaining insulin independence and maintained glycemic control. Nevertheless, this study came along with the previous study in 2007, where the research was not conducted in a random or controlled manner. Also, it had a moderately small sample size.3
Additional work was performed to learn the core mechanism of improved β cell function and to deal with the limits of earlier studies. In 2012, a medical trial was carried out with nine newly diagnosed T1D in order to evaluate the benefit of stem cell treatment, considering treatment optimization. Twelve months from post-AHST transplantation, it was identified that six patients did not depend on insulin for treatment, and three patients who still needed insulin at a lower-dose-peptide production was significantly higher in patients with insulin-independent in addition to more AHST-modified genetic proceedings. Possibly, the variance in patient reactions could be as a result of these distinct transcriptional events in the peripheral blood mononuclear cell.
Further analysis of the previous group immune immunity recommends that elimination of islet-specific autoreactive T cells could lead to enhancement of islet function following treatment in recently diagnosed T1D.16 Unlike previous researches, in the same year, a study was conducted in China using an organized study to include patients with T1D and diabetic ketoacidosis. 10 out of 13 patients, who had associated diabetic ketoacidosis, were treated with AHST treatment and followed for 31 to 54 months. It was reported that the number of CD3+ T cells and β cells were significantly reduced after AHST treatment. The reaction of these cells in some patients was inhibited by AHST as suggested by autoantibody data. Eleven of the patients needed considerably reduced insulin quantities with lower levels in HbA1c and amplified levels of C-peptide. This study further supports the effectiveness of AHST as a T1D treatment even in patients with diabetic ketoacidosis.16
Additionally, a new procedure was developed for Stem Cell Educator therapy and in this procedure, the lymphocytes in patients' blood were separated and co-cultured with multipotent stem cells derived from adherent human cord blood, whereas the blood was circulating through a closed-loop system and eventually returning to the patient’s circulation (Figure 3).18 This procedure tested 15 patients with T1D from them; 12 of the patients received the Stem Cell Educator therapy with adherent human cord blood-derived stem cells, which are multipotent, and the control group containing three patients received the Stem Cell Educator therapy, but no stem cells were used. All patients showed great treatment tolerance in the absence of any harmful effects. This procedure did not report any adverse effects of previous immunosuppression and stem cell treatments. The baseline C-peptide levels were increased after receiving stem cells, but on the other hand, the glucose-stimulated C-peptide levels were decreased. The decreased medial HbA1c values and decreased medial doses of insulin were also compared to the control group. All these parameters indicated to improved insulin production, β cell function, and glycemic control. Nevertheless, the restrictions of this work incorporated an insignificant number of the control group in comparison to the experimental group. It must be added that the entire patient sample size was minor.18

Figure 3 Summary of stem cell mentor treatment. A blood cell centrifuge (right) connected to type 1 diabetic participant (left) including the stem cell educator (bottom center) to form a system that is closed. The contributor's blood lymphocytes travel through the stem cell educator where they link up with cord blood stem cells existent in the inner surface of the device. Processed lymphocytes are returned to the patients' blood flow.8
Treatment of diabetes type 2 in clinical trials with humans:
Stem cell treatments for T2D showed its effectiveness despite the few clinical trials that have been conducted. Historically in 2008, Estrada and his colleagues worked on a study that combined intrapancreatic autologous stem cell (ASC) infusion therapy with hyperbaric oxygen treatment (HBO) in 25 patients with T2D. During the follow-up period, plasma glucose and HbA1c values declined, and C-peptide increased. Post-treatment, oral hypoglycemic drug dosage, and inoculated insulin necessities decreased. This study reported the effectiveness of ASC in combination with HBO to treat patients with T2D. Some limitations of this study include the lack of random or controlled samples, and the small sample size.3
Another study was carried out three years later to evaluate combined autologous bone marrow stem cell transplantation (ABMSCT) with HBO treatment in patients with T2D. This study had a large sample size in addition to the long follow-up period over two years. HbA1c values decreased in all patients within 30 days post stem cell treatment. Furthermore, C-peptide significantly increased within 90 days post-treatment, but at other points in the follow-up, levels were likewise to baseline. Remarkably, hypoglycemic drug dosage and exogenous insulin dosage significantly reduced in all patients. The study found that the pancreatic β cell functional progress may be transient despite combined stem cell therapy, and HBO treatment in patients with T2D can recover glycemic control, and lessen insulin and hypoglycemic drug reliance. Similarly to the previous work, this study was also not randomized or controlled and had a small sample size.3
A year later, a large organized clinical trial on bone marrow mononuclear stem cell treatment for T2D was conducted. This study included 118 patients who divided into experimental and control groups based on stem cell retrieval receiving stem cell treatment, so the study was not random. During the follow-up period (36 months), the experimental group (52 patients) received autologous bone marrow mononuclear cell embedding, whereas the control group (62 patients) received insulin intensification therapy.19 The experimental group displayed decreased mean fasting plasma glucose, whereas the control group showed increased fasting plasma glucose. This explains the elevation in insulin dosage in the control group. Therefore, fasting plasma glucose is almost the same in the two groups. 18 out of 56 patients, in the experimental group, showed a significant decrease in insulin dosage per day, achieving insulin independence as compared to the control group. For BMI, there was no substantial variance between the two sets. However, C-peptide was essentially raised from the baseline for the experimental group. Moreover, HbA1c levels showed a significant difference between the two groups at the end of the first year. These findings indicated the benefit of bone marrow mononuclear stem cell treatment for T2D.19
In 2014, Bhansali and his colleagues conducted the most recently reported clinical trials using autologous bone marrow-derived stem cell transplantation (ABMSCT) in 21 patients with T2D. This study had randomized and controlled groups and initially was assigned the 21 patients to trial and control groups. The follow-up study was carried out on ten patients. It was found that inulin dose was significantly different between the experimental and the control groups at 3, 6, and 12 months post-treatment (20). Additionally, the experimental group showed a 66.7% decline in insulin needs at 12 months. In this study, a positively and significantly association was reported between the reduction in insulin requirements and the increase in C-peptide. Between the two groups, no important alterations were found in HbA1c; however, 10 out of 11 experimental group patients reached HbA1c levels <7%, whereas only 6 out of 10 control group patients had done at 12 months. Moreover, 15 months post-treatment, it was found that the mean insulin equipment decreased in all patients. All patients showed improvements in blood pressure and high-density lipoprotein cholesterol (HDL), and the need for insulin decreased to about >50% in the majority of patients. Furthermore, there were no side effects of ABMSCT treatment in this study. These findings showed the importance of ABMSCT as a potential treatment for T2D. The limitations of this study included the absence of randomized and controlled samples, the shortness of follow up time, in addition to the small sample size.20 The human clinical trials are presented in Table 1, whereas the current trails are shown in Table 2.
Authors |
Year Published |
Sample Size |
Patients (Sample type) |
Study Protocol |
Follow-up Duration (time) |
Treatment Conclusion |
Voltarelli et al. |
2007 |
15 |
Newly diagnosed patients with type 1 diabetes |
High Dose Immunosuppression and AHST |
7-36 months (mean = 18.8) |
Improved beta cell function in all patients except one patient with insulin dependent and acceptable toxicity |
Couri et al. |
2009 |
23 |
Newly diagnosed patients with type 1 diabetes |
High Dose Immunosuppression and AHST |
5-58 months (mean = 29.8) |
Increased C-peptide levels in most patients who have insulin independence and glycemic control |
Estrada et al. |
2008 |
25 |
Type 2 diabetics on metformin or combination of OHAs |
Hyperbaric oxygen treatment combined with ABMSCT |
12 months |
Decreased oral hypoglycemic drug dosage and injected insulin requirements |
Wang et al. |
2011 |
31 |
Type 2 diabetics, failure of triple OHA therapy & insulin dependent |
Hyperbaric oxygen treatment combined with ABMSCT |
10 months |
Improvement in glycemic control, insulin/ oral glycemic drug requirements and beta cell function transiently |
Zhaog et al. |
2012 |
15 |
Type 1 diabetes |
Stem Cell Educator: immune modulation by human cord blood-derived multipotent stem cells |
40 weeks |
No side effects reporting forms besides increased C-peptide levels, decreased HbA1c values and medical doses of insulin |
Hu J., et al. |
2012 |
118 |
Type 2 diabetics on metformin, rosiglitazone and insulin dependent |
Exp: ABMSCT |
33 months |
Safe and effective and partially restored the function of b cells and blood glucose homeostasis |
Control: insulin intensification treatment |
||||||
Li L., et al. |
2012 |
13 |
Newly diagnosed patients with type 1 diabetes |
AHST |
60 days |
Decreased insulin doses with decreased levels in HbA1c and increased levels of C-peptide |
Zhao, X. et al. |
2012 |
9 |
Newly diagnosed patients with type 1 diabetes |
AHST |
23 months |
Improved beta cell function and a proposed mechanism that could have an elimination of islet-specific autoreactive T cells |
Bhansali A., P. Asokumar, et al. |
2014 |
21 |
Type 2 diabetics with triple oral antidiabetic drug failure and insulin dependent |
ABMSCT |
12 months |
Decreased insulin requirements and increased C-peptide levels |
Bhansali A., V. Upreti, et al. |
2014 |
10 |
Type 2 diabetics with a >5-year duration of disease with documented triple drug failure, receiving metformin and pioglitazone, and insulin dependent |
ABMSCT |
15 months |
Positive therapeutic effects of bone marrow mononuclear stem cell treatment |
Table 1 Summary of discussed human clinical trials3
Condition |
Intervention |
(Age, Gender) |
Location (s) |
Title of the research |
Type 2 diabetes |
Procedure: Harvesting and Implantation of Adipose-Derived Stem Cells (ASCs) |
18-80 years, both genders |
Miami, FL, USA |
Safety and Effects of Autologous Adipose-Derived Stromal Cells Delivers in Patients with Type II Diabetes |
Diabetes |
Procedure: Valuation of therapy impediments Other: laboratory biomarker examination |
18 years and older, both genders |
Nashville, TN, USA |
Predicting Development of Diabetes Mellitus in Patients Undergoing Allogeneic Stem Cell Transplant |
Type 2 diabetes |
Drug: Saxagliptin Drug: placebo |
40 -70 years, both genders |
Washington, DC, USA |
Effect of Saxagliptin on EPCs as a Cellular Biomarker for Evaluating Endothelial Dysfunction in Early Type 2 Diabetes |
Type 1 diabetes |
Procedure: Immunosuppression and stem cell transplantation |
8-35 years, both genders |
Nanjing, Jiangsu, China |
Efficacy and Safety Study of Autologous Hematopoietic Stem Cell Transplantation to Cure New-Onset Type 1 Diabetes |
Type 1 diabetes |
Device: Stem Cell Educator |
14-60 years, both genders |
Shijiazhuang, Hebei, China. Changsha, Hunan, China. Jinan, Shandong, China. Oviedo, Asturias, Spain |
Stem Cell Educator Therapy in Type 1 Diabetes |
Type 1 diabetes |
Biological: Autologous mesenchymal stem cell transplantation |
18-40 years, both genders |
Uppsala, Sweden |
Mesenchymal Stem Cells to Intervene in the Development of Type 1 Diabetes: A Blinded Randomized Study |
Type 2 diabetes |
Biological: Umbilical cord mesenchymal stem cells Biological: Organized suspension liquid |
20-60 years, both genders |
Beijing, China |
Mesenchymal Stem Cells to Treat Type 2 Diabetes (UC-MSCs) |
Diabetes insulin dependent |
Biological: Intravenous mesenchymal stem cell infusion |
12-35 years, both genders |
Ribeirão Preto, São Paulo, Brazil |
Safety and Efficacy Of Mesenchymal Stem Cells in Newly-diagnosed Type 1 Diabetic Patients |
Type 1 diabetes |
Biological: Autologous transplantation |
10-40 years, both genders |
Chongqing, China |
Autologous Transplantation of Mesenchymal Stem Cells in treating Type 1 Diabetes Mellitus |
Diabetes |
Other: Intra thecal transplantation of autologous mono nuclear cells |
18-55 years, both genders |
Pune, Maharashtra, India |
Study Safety and Efficacy of Bone Marrow Derived Autologous Cells for the Treatment of Diabetes Mellitus (BMACD) |
Type 2 diabetes |
Other: Bone Marrow Mononuclear Cell Transplantation |
30-70 years, both genders |
Beijing, China |
Autologous Bone Marrow Mononuclear Cell Transplantation in Treating Type 2 Diabetes Mellitus |
Type 1 diabetes |
Device: Stem Cell Educator |
6-14 years, both genders |
Changsha, Hunan, China |
Reversal of Type 1 Diabetes in Children by Stem Cell Educator Therapy |
Type 1 diabetes |
Genetic: Stem Cell Educator Therapy |
18 years and older, both genders |
Hackensack, New Jersey, USA |
A Pilot study of the Therapeutic Potential of Stem Cell Educator Therapy in Type 1 Diabetes |
Table 2 Existing human medical experiments for stem cell interference in diabetes cure3
Challenges:
The outstanding problem in stem cell research is the production of transplanted stem cells from immune attack despite the progress reported in this area. Even in T1D that is caused by an autoimmune disease leading to β cell destruction, patients should receive immunosuppressant drugs to avoid a response to the new stem cell-derived β cells. The adverse effects of immunosuppressed drugs include various complications such as pneumonia and oligospermia, as referenced in the previous studies.21,22 Recently, ViaCyte, a regenerative medicine company located in San Diego, developed a potential solution to immunosuppression, which is encapsulated and biocompatible cells. Despite recent progress in animal studies, the body shows the capacity to form scar tissue around external bodies preventing the enclosed cells from receiving nutrients. Though, patients with diabetes prefer to take immunosuppressant rather than insulin injection if stem cell therapy is additionally improved and studied in randomized and controlled trials despite these challenges.3,23
Stem cell therapy can be used in the future as the potential treatment for patients with diabetes and may provide a more permanent solution to control hyperglycemia. This was confirmed in human trials through the increased C-peptide levels and decreased HbA1c levels, proving that a practical approach towards diabetes is provided by stem cell therapy. Those trials were carried out using various stem cells in humans and showed positive results. However, there were some limitations that require further studies using more extensive and better-controlled experiments. Moreover, future studies require randomizing human trials. Additionally, comparative studies are needed to decide the method, and the stem cell type produces the best result for human patients. In conclusion, embryonic and adult stem cells could both be used as an excellent resource to distinguish to insulin-producing cells. However, adult stem cells could offer therapy to more patients. This is due to easy accessibility and less controversy in nature in comparison to embryonic stem cells. Furthermore, many patients feel more comfortable being treated by their tissue, showing faster treatment and more effective by this method. Further clinical trials on stem cell therapy are required to confirm the continued progress for treating diabetes in the future.3,24
This paper was supported by the molecular medicine department in the College of Medicine and Medical Sciences, Arabian Gulf University, Kingdom of Bahrain.
The authors declare no conflict of interest.
First author: Haifa Abdullah, writing the manuscript. Second Author: Sfoug Alshammary, Reviewing and supervision

©2020 Abdullah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.