Journal of
eISSN: 2475-5540


Review Article Volume 5 Issue 2
Department of Biological Sciences, USA
Correspondence: Vincent S Gallicchio, Department of Biological Sciences, College of Science, Clemson University, Clemson, SC 29637, USA
Received: April 23, 2019 | Published: April 30, 2019
Citation: Chase DM, Gallicchio VS. The effect of mesenchymal stem cells and exosomes to treat idiopathic pulmonary fibrosis. J Stem Cell Res Ther. 2019;5(2):48-59 DOI: 10.15406/jsrt.2019.05.00134
Idiopathic Pulmonary Fibrosis (IPF) is a disease that consists of the scarring of the lungs. It is the most common type of pulmonary fibrosis. This disease is irreversible and becomes worse over time. In patients with IPF, treatment relies mostly on the clinical application of new drugs. Unfortunately, these drugs do not repair damaged lung tissue; therefore, these medications only have the ability to slow down disease progression. With this dilemma, stem cell treatment has become a popular alternative in the treatment of IPF, specifically mesenchymal stem cells (MSCs). MSC therapy would repair damaged lung tissue, thus not delaying the progression of the disease, but instead repairing the lungs of the patient. In addition, the application of exosomes has also gained popularity because of their functionality in intracellular communication. There is a need for regenerating the damaged lung tissue of patients with IPF, which can be accomplished with stem cell therapy. The clinical application of MSCs has been proven safe in patients with this degenerative disease, thus this finding has justified more research for the application of stem cell therapy in patients with IPF.
Key words: idiopathic, pulmonary, fibrosis, exosomes, stem cells, irreversible
IPF, Idiopathic Pulmonary Fibrosis; AECs, Alveolar Epithelial Stem Cells; IIP, Idiopathic Interstitial Pneumonias; FVC, Forced Vital Capacity; MSC, Mesenchymal Stem Cells; HLA, Human Leukocyte Antigen; BLM, Bleomycin; hMSCs, Human Mesenchymal Stem Cells; BMSCs, Bone Marrow Mesenchymal Stem Cells; BMP-7, Bone Morphogenetic Protein-7; STC1, Stanniocalcin-1; ADSCs, Adipose-Derived Stem Cells; HGF, Hepatocyte Growth Factor; FDA, Food and Drug Administration; HUC-MSC, Umbilical Cord-Derived Mesenchymal Stem Cell; LTOT, Long-Term Oxygen Therapy; ATII, Alveolar Type II; miRs, MicroRNA; BALF, Bronchoalveolar Lavage Fluid; EVs, Extracellular Vesicles; hAEC Exo, Human Amnion Epithelial Cell-Derived Exosomes; HLF, Human Lung Fibroblasts; MEx, Mesenchymal Stem Cell Exosomes; hAECs, Human Placental Amniotic Epithelial Stem Cells
Idiopathic pulmonary fibrosis (IPF) is a progressive type of lung disease that involves lung tissue becoming scarred thus inhibiting the lung’s ability to function properly.1 The scarring’s location normally progresses from the edge of the lungs toward the center as well as increasing in the amount of scarring.2 Over time the closer the scarring gets to the center of the lungs as well as the amount of scarring in the lungs reduces pulmonary function more difficult to breathe and deliver necessary oxygen throughout the body in patients with IPF.2
IPF is a debilitating condition that is irreversible and unfortunately has few treatment options.3 The cause of this debilitating disease is unknown; however, exacerbating the clinical condition is the fact that the lungs are the only internal organs with direct exposure to the external environment. Thus, the lungs are exposed to a variety of elements, which could be possible risk factors.2 Therefore, the cause of IPF could involve genetic, environmental or toxic components, but it remains unclear what is the main cause of the disease.4
Because IPF has limited treatment options, stem cell therapy has begun to be a potential option for lung tissue regeneration. Throughout life, animals depend on stem cell populations to maintain and repair their tissues to ensure life-long organ function. This is because stem cells have the capacity to self-renew and give rise to differentiated cell types. Stem cells possess the necessary properties required to address the needs for tissue replacement to maintain normal lung homeostasis as well as organ tissue regeneration after lung injury. The capacity of organ tissue regeneration of the lung epithelial stem cells involves communication with their immediate microenvironment. Thus, it is this local tissue environment that influences stem cell behavior of in part because the tissue environment is comprised of other cell types and the extracellular matrix. To have a regenerative response after lung injury, cross-talk needs to occur between the epithelial-mesenchymal stem cells as well as the signaling from fibroblast growth factor. When these communications and interactions are disrupted, cellular dysfunction can occur and may result in chronic lung diseases such as IPF. Furthermore, current research indicates that the fibrotic response of patients with IPF may be due to abnormally activated alveolar epithelial stem cells (AECs). Patients with IPF have an increase in epithelial stem cells despite the lung tissue damage.5 AECs produce mediators that bring about the formation of fibroblasts and myofibroblasts through the proliferation of resident mesenchymal cells and the attraction of circulating fibroblasts. It is the fibroblast and myofibroblast that secrete excessive amounts of extracellular matrix that results in the scarring and destruction of the lungs.6 This dysfunction leads to abnormal stem cell activation with stem cell loss which prevents proper regeneration and leads to permanent tissue damage. Therefore, stem cells are vital during normal cellular homeostasis and regeneration. A better understanding of stem cells microenvironments and their regulatory pathways involved will lead ways to recreate the microenvironments and develop cell replacement therapies.5
IPF affects about 50,000 people in the United States and about 3 million people worldwide. It is estimated that there are 15,000 new cases of IPF developing annually in the United States.2 IPF occurs in mainly older adults with the median age being at 66 years with a range of 55-75 years.4 This pathological lung condition is the most common and severe type of idiopathic interstitial pneumonias (IIP). IPF mortality rates remain high with a 50% mortality rate three years after diagnosis.3 The average survival of patients with IPF being 2–5 years from the onset of symptoms.7
As stated before, IPF is a degenerative disease of pulmonary tissue having little to no effective treatment. Pirfenidone was the first medication used in Japan to treat patients with IPF. However, it could not regenerate damaged tissue and only suppressed the disease.3 In addition, nintedanib is another antifibrotic drug that has also been shown to reduce the decline in forced vital capacity (FVC), decrease exacerbation, and improve mortality rates in several studies.4 According to Barczyk et al.8 despite these drug benefits, the medication did not improve the patients’ quality of life and survival. Drugs such as these only slow down the progression of IPF, unfortunately they do not reverse the fibrosis process.4 Thus, the only practical way to regenerate damaged pulmonary tissue would be with stem cell therapy.3 With this goal in mind, there is new evidence that the administration of mesenchymal stem cells (MSCs) could be an effective treatment for IPF patients based on the rationale that MSCs are known for their ability to differentiate into a variety of cell types, thus, they can migrate to and repair damaged tissue in anatomical locations, which would make stem cell therapy an effective use for reversing IPF.7 Stem cells have the high capacity to differentiate into special tissues which makes stem cell therapy a popular candidate for degenerative diseases.3 In this review, new evidence regarding the use of mesenchymal stem cells and exosomes will be discussed as an effective treatment to patients with IPF.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are self-renewing, multipotent progenitors that can be isolated from a variety of tissues.9 They are also easily accessible and can be simply collected from a variety of tissues such as adipose tissue, umbilical cord blood, liver, amniotic fluid, placenta, and other tissue sources. MSCs have the capability to differentiate, promote tissue-repair, immunosuppression, etc., thus they are an ever growing clinical therapeutic option for potential testing in clinical trials.9 MSCs are among the best investigated stem cell populations. Bone marrow derived MSCs are the most frequently studied of this type.10 As shown in Figure 1, MSCs are characterized by a variety of properties. Additional properties present to allow for the potential therapeutic effects of MSCs include low immunogenicity and transplantation in vitro, immunomodulation and tissue repair, and self-proliferation.11 Based on their anti-inflammatory, antifibrotic, antiapoptotic, and regenerative properties, MSCs are widely considered for the treatment of lung diseases such as IPF.12
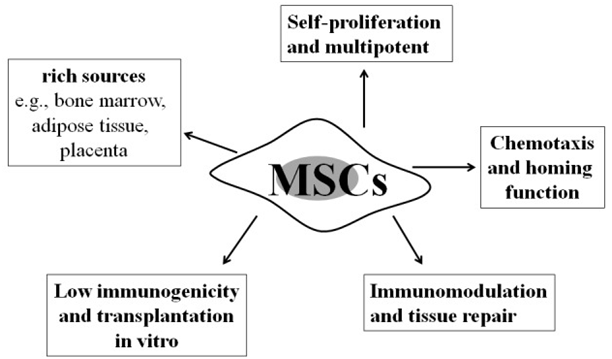
Figure 1 This chart shows all of the properties of mesenchymal stem cells (MSCs). With further research in MSC transplantation, these stem cells could be crucial in treating idiopathic pulmonary fibrosis (IPF).11
In the lungs, MSCs contribute to tissue regeneration after elastase-induced emphysema, tissue remodeling in a rat monocrotaline model of pulmonary hypertension, and restoring chronic airway inflammation. MSCs have the potential to also modulate the fibrotic response to radiation-induced injury.13 MSCs may be able to home to sites of injury where they exert anti-inflammatory effects, differentiate into local cell types, and could activate local resident stem cells.13 They have the ability to target the sites of injury to produce an increase in lung function.12
MSCs are thought to lead to improved lung compliance and gaseous exchange.13 Resident mesenchymal cells such as pericytes have been demonstrated to give rise to the vast majority of myofibroblasts. This observation leads to suggesting that lung-resident MSCs contribute to the disease process. MSCs show multipotency which means they are able to give rise to several cell types within the mesenchymal lineage. Another benefit of MSCs is their lack of human leukocyte antigen (HLA) expression, this is called the “immune-privileged status” of MSCs. This makes MSCs the ideal candidate for cell transplantation since HLA incompatibility is not an issue.10 MSCs have been studied as potential therapeutic agents for lung diseases because of their tissue reparative and immunomodulatory properties.14
Preclinical studies
Mesenchymal stromal cells are a key component of the stem cell niche in bone marrow and other body organs such as the lungs. They possess this key component because they have been shown to enhance epithelial repair and have also been shown to be effective in induced pulmonary fibrosis preclinical models.15 MSCs remain in pulmonary tissue longer than other organs. In bleomycin-induced mice, MSCs were found to exert a protective effect by improving inflammation and reducing the injury and fibrosis. Also, MSCs produce growth factors that are implicated in epithelial repair.16 MSCs have been tested in experimental systems. The administration of MSCs was given to normal mice compared to BLM-induced mice and the results showed that it is an effective method to treat lung disease. MSCs immediately protected the lung tissue from the injury in animals by reducing inflammation, collagen deposition, and fibrosis. MSCs used in this study were from plastic adherent cultures of murine bone marrow which contain many hematopoietic cell types. The MSCs may replace the alveolar epithelial type II cells that are thought to function as stem cells in the lung. As MSCs differentiate into these cells, they may partially restore the stem cell pool thus repairing the tissue. This data in the report indicates MSC administration can ameliorate injurious effects of BLM. Furthermore, MSCs reduced inflammation and collagen deposition seven days after BLM instillation.17
Similarly, another study showed MSCs can migrate into damaged lung tissue and develop functional characteristics of alveolar cells, which would slow down the progression of the disease. They completed this study by using BLM-induced pulmonary fibrosis in a rat model. As a result, lungs of rats with BLM-induced lung disease showed prominently smaller pulmonary alveolus cavities when compared to the lungs of the control group. This proved the differentiation capability of these stem cells does exert a therapeutic effect. This study also showed MSCs are only effective to provide this capability within the microenvironment at the site of pulmonary injury. The study showed MSCs are able to repair the damage caused by the BLM injury and does this by blocking oxidative stress. The results also showed the reduction of collagen deposition after BLM instillation. These results collectively demonstrate the need for further studies that confirm MSC migration and differentiation will provide an effective cell therapy for pulmonary fibrosis.18 Another study also reported their findings on BLM-induced fibrosis in mice immediately after the administration of BLM. Investigators found MSCs protect lung tissue, blocking the pro-inflammatory cytokines, TNF-a and IL-1.19 Rojas et al.,16 also reported the induction of allogeneic BM-MSCs to a BLM induced mouse and proved no adverse effects. The results concluded with reduced proinflammatory cytokines and improved survival. Bleomycin (BLM) is commonly used to induce idiopathic pulmonary fibrosis symptoms in animal models. This is because of the oxidative stress it produces is specific to alveolar epithelial cells.4
An animal study using IPF model mice was performed to provide evidence that transplanted mesenchymal stem cells can directly replace fibrosis with normal lung cells (3). This study used bone marrow-derived human mesenchymal stem cells (hMSCs) which were cultured in high glucose Dulbecco’s modified Eagle’s medium with 20% fetal bovine serum and used microvesicles that were isolated from this media by using ExoQuick (3). Two groups demonstrated the IPF model, female mice were injected with hMSCs or microvesicles released from hMSCs.3 Reporters measured the wet/dry weight of whole lungs. They showed the transplantation of hMSCs into treated mice reduced the wet/dry ratio meaningfully, but there was no significance of lung weight. The microvesicle transplanted group did not show much significance. In addition, the degree of lung fibrosis was evaluated in hMSCs or microvesicle treatment. After 12 and 14 weeks, they found a decrease in inflammation and density. Therefore, transplantation of hMSCS significantly reduced every aspect of IPF. They also showed the ability of these cells to essentially differentiate into functional lung cells by using RT-PCR. They successfully found a marker of differentiated lung II cells in the hMSC transplanted mouse lung confirming the differentiation. Moreover, the microvesicles were shown to reduce airway inflammation and collagen deposition as well as reduce silica induced pulmonary fibrosis.3
Another report focused on the safety and efficacy of HUC-MSCs in rat liver fibrosis. Investigators found that the fibrosis decreased in 8 weeks after stem cell transplantation. This study was from the same authors who showed the efficacy and safety of HUC-MSCs in humans discussed subsequent. This proves that HUC-MSCs are capable of attenuating fibrotic processes in animals such as mice as well as clinically.20
Often in animal studies, the use of silica exposure on a mouse ultimately results in defuse pulmonary fibrosis. This is referred to as silicosis, which is an occupational lung disease in humans caused by the introduction of those silica particles.21 A report highlighted that bone marrow mesenchymal stem cells (BMSCs) attenuated silica-induced fibrosis and reduced the injury of the alveolar epithelial in vivo and in vitro. They investigated the anti-fibrotic effect and mechanisms of these stem cells, finding the effects may be because of paracrine rather than differentiation. The rats of this experiment were exposed to silica in vivo and in vitro. After 15 or 30 days of intravenous injection of BMSCs, they were examined. It showed that BMSCs decreased the blue areas of collagen fibers and the number of nodules. The alveolar epithelium was damaged by the silica given and then the BMSCs restored the tissue. The results of the study found the anti-fibrotic effects of the stem cells on the silica-induced pulmonary fibrosis, and showed it may be because of the paracrine mechanisms not the differentiation ability.22 In another study, the exposure of silica was used and evidence reported the anti-fibrotic role of bone morphogenetic protein-7 (BMP-7) and BMSCs in lung disease. Here, they modified BMSCs to overexpress the BMP-7 gene by lentivirus transduction. Investigators compared four groups of rats that included a control group, silica, BMSCs, and BMP-7-BMSCs. After examination of the rats after 15 or 30 days treatment, histopathological results showed that the BMP-7-BMSCs group and the BMSCs group exhibited remarkable blockage of the progression of the silica-induced fibrosis. They also reported that the anti-fibrotic effect was enhanced by BMP-7 which could be a potential therapeutic intervention for this disease.21
A study showed that MSCs are able to correct the poor communication between epithelial and mesenchymal cells through Stanniocalcin-1 (STC1) secretion. When using a STC1 plasmid, the study showed the increase of the MSCs ability to ameliorate the fibrosis. STC1 has antifibrotic effects and tends to correct the miscommunication between epithelial and mesenchymal cells, thus these cells could be an effective treatment for IPF. The STC1 would need to block this miscommunication to treat this disease. This was completed by using a bleomycin-induced IPF model. STC1 was injected via the trachea or the tail vein and the lungs were observed at day 14. Remarkable pathological improvements were observed in the STC1 and hMSCs treated groups compared to the control group. The hMSCs were shown to ameliorate lung injuries because of the presence of STC1. This study showed that MSCs enhance STC1 secretion. Once the levels of STC1 are adequate, then they can diminish oxidative stress, confirming that STC1 is an important player for MSCs to protect the lung against injuries and fibrosis in mammals. The same report investigates the effect of STC1 on BiP expression in alveolar epithelium cells. Investigator conducted studies found the results suggesting MSCs reduced the endoplasmic reticulum stress in the lung microenvironment through paracrine secretion of STC1. Furthermore, this study confirmed that human and mouse MSCs reduce collagen deposition and oxidative stress in the BLM-induced pulmonary fibrosis model. However, they found that this result relies on the secretion of STC1 from that mesenchymal stem cells.23
Another study observed the effects of adipose-derived stem cells (ADSCs) to be effective in lung repair and regeneration. In this report, IPF was induced in mice by intratracheal instillation of bleomycin and the stem cells were delivered systemically into the mice via tail vein. Adipose-derived stem cells are more abundant and widely available compared to bone marrow derived stem cells and importantly they produce similar properties. These properties include multiple differentiation potential, high proliferation, and immunoregulatory ability. From this study, using mice indicated that ADSC intervention alleviates IPF. The stem cells do this by decreasing the apoptosis of alveolar epithelial cells and decreasing the production of proinflammatory factors. These results showed ADSCs may relieve IPF and provide lung repair when given at an early stage of life.24
Following these preclinical trials, it was shown that MSCs demonstrate a great capability in the realm of tissue repair and fibrosis. However, there is a need for animal models more representative of chronic IPF. This is due to the recent criticism that bleomycin induced pulmonary fibrosis reaction to therapies may be resulting from prevention of cascade rather than reversal of fibrosis. The use of different animal models would clear these disprovals and enable biomarker identification to use for measures of disease activity or the effect of the treatment. It is also stated that the clarification of many factors needs to be further explored. This includes the timing of the administration of the cells, the age of the mice, etc.25
Clinical trials
In clinical trials for IPF, drugs were found to be ineffective or even potentially harmful. These negative results were from medications such as Carlumab, NAC, Sildenafil, Triple therapy, etc. There were harmful effects from using a triple therapy of prednisone, azathioprine and NAC. In addition, rapid progression of the disease occurred in these studies with endothelin-1 receptor antagonist ambrisentan, the anti-proliferative drug everolimus, or warfarin. As stated before, the only drugs approved in the United States to treat IPF are pirfenidone and nintedanib. Several ongoing studies are being designed for other drugs to treat IPF. This includes thalidomide, omeprazolde and azithromyocin. These drugs have been used to improve chronic intractable cough which is a symptom of IPF. While pharmacological compounds in clinical studies are being designed, mesenchymal stem cells studies are ongoing. Mesenchymal stem cells (MSCs) produce an anti-fibrotic effect on injured lungs. This is partly due to the secretion of hepatocyte growth factor (HGF) or through the upregulation of the expression of endogenous HGF and prostaglandin E2. Growth factors are known to contribute to re-epithelialization. Furthermore, using MSCs for stem cell therapy is beneficial because these stem cells are retained in the lungs before migrating to other organs and they stay in the lungs longer.16 Clinical studies of MSCs in patients with this disease have shown promising results. They have shown the safety for stem cell therapy using MSCs with patients with IPF. In addition to the safety of the administration of MSCs, they have also shown the improvement of the quality of life in patients.25 The studies listed and explained below mostly evaluate the safety and tolerability of the administered cells as well as the potential benefits of stem cell therapy to treat this degenerative disease.
In a study with the transplantation of allogeneic bone marrow mesenchymal stem cells in IPF patients, they were assigned three cohorts to a single IV infusion of 20, 100, or 200x106 human BMSC.4 To support the safety of a single infusion of human mesenchymal stem cell delivery, Glassberg et al.,26 performed this trial with nine patients with mild to moderate IPF. This report set up and experiment is represented in Figure 2. They began with eleven patients but two participants withdrew before treatment. The report states that this trial using allogeneic human stem cells (hMSCs) in IPF patients via intravenous delivery (AETHER) trial was the first study designed to evaluate the safety of it. The nine patients were dosed with single infusion of bone marrow-derived hMSCs from young, unrelated, men. The results concluded that no serious adverse events were reported. By 60 weeks after infusion, there was a 3.0% mean decline in % predicted forced vital capacity (FVC) and 5.4 % mean decline in % predicted diffusing capacity of the lungs for carbon monoxide.26 These results justified the safety of intravenous infusions of allogeneic bone marrow-derived human MCSs in IPF.14 The authors of this study believed that despite the Food and Drug Administration (FDA) approval of the two new drugs, pirfenidone and nintedanib, curative therapies remain elusive and mortality remains high.4 This trial provided an important step into the evaluation of these stem cells to treat this disease. Although, more information and studies are needed to confirm the complete treatment of this therapeutic option.27
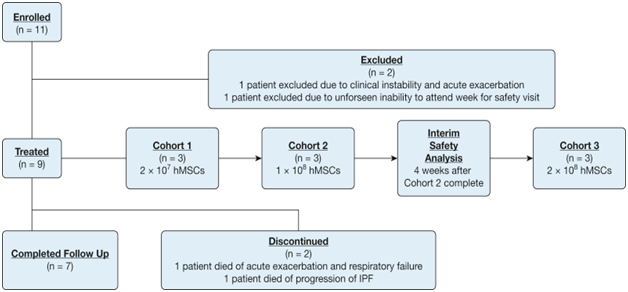
Figure 2 The flow chart represents the clinical trial performed by Glasberg et al.26 This chart represents the participant flow chart and how the experiment was produced, step by step.
Next, there was also a case report at the Siping Hospital of China Medical University that describes a 56-year-old man with IPF who tolerated umbilical cord-derived mesenchymal stem cell (HUC-MSC) intravenous infusion. The background of this patient includes his previous diagnosis of COPD for five years before being diagnosed with IPF, as well previously smoking cigarettes. HUC-MSCs were used because of their high capacity of proliferation, widespread availability, convenience and relatively cheap cost. The HUC-MSCs that were used came from two donated umbilical cords from healthy mothers after a cesarean section birth and the cells were isolated and propagated. After perfusion of the HUC-MSCs into the right median cubical vein, the patient was monitored and discharged then assessed at 6 and 12-month post-transplantation. As a result of the therapy, long-term oxygen therapy (LTOT) was reduced and oxygen was not required at the end of two months. This decline may have been due to the strengthening of the patient’s respiratory muscles thus improving his physical performance and quality of life. In the 12-month post transplantation, there were increases in lung function and CT scores and decreases in the fibrosis area. There were no serious side effects in the 12 months from this study which shows the safety and efficacy of HUC-MSCs to treat IPF in the short-term. In addition, at 12 month post-infusion, lung function, 6MWD and CT fibrosis score were all increased from baseline. The study identified the long-term safety of stem cell therapy for patients with IPF. As depicted in Figure 3, it shows a radiological scan before and after the HUC-MSC treatment at the end of 12 months. The fibrosis area decreased after transplantation of these stem cells.20
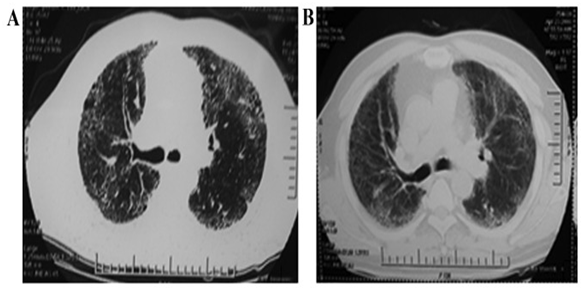
Figure 3 A radiological scan showing the effects of HUC-MSC treatment before and after 12 month follow-up. A represents before the HUC-MSC treatment and B represents the scan at 12 months after stem cell treatment.20
An additional study published that is in the phase Ib is designed to show the safety of autologous adipose-MSCs to mild to moderate IPF patients. With the dosage of 0.5x106cells/kg of body weight, these autologous MSCs were isolated from adipose tissue and delivered into 14 patients. They did this through endobronchially administration and confirmed the safety of it. After following up 12 months after the administration of the cells, they found that adipose-MSCs were safe and no deterioration of functional parameters and indicators of quality of life were observed.28 Therefore, no cases of serious or clinically meaningful events were included in the short term and long term for any of the patients.29
Next, another clinical study used heterologous placenta-MSCs to eight patients with mild to moderate IPF via a peripheral vein. Two doses of cells for this trial included 1x106 or 2x106cells/kg of body weight. These patients were monitored for six months. This study design includes being a phase1b, non-randomized, and dose escalation trial. The results concluded that there was not any evidence of the fibrosis worsening, which shows MSC administration is feasible and has a good short-term safety. The patients tolerated the administration well with only minor and transient acute adverse effects after a 6 month follow up.15
In addition, another clinical study tested alveolar type II (ATII) cells to investigate the safety and tolerability of them in patients with IPF. Embryonic stem cells and induced pluripotent stem cells (iPSCs) differentiated into alveolar type II cells can be delivered intravenously or intratracheally. These cells will home to the sites of injury in the lungs. Once these cells are administered, they can exert anti-inflammatory and anti-fibrotic effects. In addition, these cells also have the capability to engage in paracrine signaling and immunomodulation, differentiate into local cell types, and activate resident stem cells that can enhance lung regeneration (Figure 4). Therefore, these cells were used clinically to determine the potential treatment for lung tissue regeneration. There were 16 patients who were administered these cells through intratracheal transplantation. The patients were followed up for 12 months, assessing any adverse side effects. The results displayed none of these events and showed no deterioration in pulmonary function, respiratory symptoms or disease extent. Furthermore, this study agrees with the safety of the ATII-cell intratracheal transplantation.30
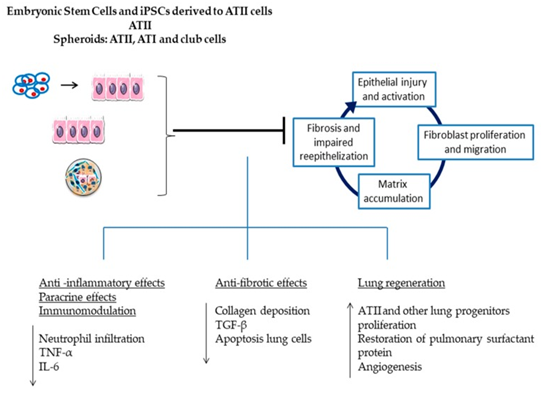
Figure 4 Alveolar type II cells (ATII cells) can be derived from embryonic stem cells and induced pluripotent stem cells. These cells have a variety of effects on the lungs. These include anti-inflammatory effects, paracrine effects, immunomodulation, anti-fibrotic effects, and lung regeneration.30
Human trials have been delayed due to safety risks and a limited knowledge of the mechanism of action of the various stem cell therapies. These risks include pulmonary embolism and inappropriate proliferation, differentiation, or homing of the systemically administered cells.14
Exosomes
Exosomes are most importantly known for the fact that a great deal of cell to cell signaling takes the form of an extracellular vesicle (EVs) such as an exosomes.31 Exosomes are extracellular vesicles that used to be thought to be necessary to remove unneeded proteins from cells. However, they found that a variety of cell types secrete exosomes including stem cells.32 These exosomes can then be harvested and employed as a therapy.31 They are important mediators of intercellular communication.33 Exosomes are generated by inward budding of the membrane through endocytosis, subsequent forming of multivesicular bodies, and released by exocytosis. The number and types of EVs released are unique according to the pathophysiological status of the disease. This is why they can be great biomarkers for many respiratory diseases.34 They can be isolated by ultracentrifugation, high-performance liquid chromatography, ultrafiltration, and volume-excluding polymers. Exosomes are able to have specific interactions with targeted recipient cells, enabling them to perform cell-to-cell communication between large distances of the body. Their functions are dependent on the components that they contain which include proteins and RNAs.32 They carry and transfer a wide variety of molecules such as microRNAs, messenger RNAs, DNA, and proteins that contribute to the physiological functions and pathology of a variety of diseases.33 Recent studies provide evidence that extracellular vesicles promote the pathogenesis of respiratory diseases by promoting inflammation and immune activation.35 Inflammation plays a role in pulmonary fibrosis. It is likely that exosomes participate in the process of tissue repair and fibrosis since evidence supports the potential for them to alter gene programs and induce differentiation or de-differentiation of target cells.35
Exosomes play a large role in homeostatic regulation and cellular function of host tissues and organs. As stated before, their main role is in intercellular communication and signaling. They can signal immune cells to modulate their functionality. Injured cells can use exosomes to signal stem cells to induce migration and differentiation to the sites of tissue injury. One report states that radiation-injured whole lungs cells can achieve a process that is bidirectional. This is where stem cells can secrete EVs that contain miRNA and mRNA that overall induce tissue repair. They do this by secreting microvesicles that can induce a specific gene in the lung and protein expression in ex vivo co-cultured bone marrow cells. Similarly, this was supported by in vitro studies that showed embryonic stem cells can secrete EVs that reprogram hematopoietic progenitor cells through the transfer of mRNA.35
Exosomes allow communication between distal cells and their cargos. They are associated with the modulation of the immune response and suppression of macrophage phenotype. In a study, they purified exosomes from amniotic stem cells and administered them intranasally. The results showed that pulmonary capacity was recovered in the exosome-treated groups in BLM-induced pulmonary fibrosis, thus displaying the potential of MSC therapy is positive.4
As stated previously, MSCs have been a popular research focus in recent years. There is evidence that shows the MSCs act in a paracrine manner so biological factors in conditioned medium, including exosomes, derived from MSC cultures are being explored. Exosomes are secreted by all cell types and are found in body fluids such as blood, urine, and breast milk. Studies have stated that MSC-derived exosomes have functions that are similar to these stem cells such as repairing tissue damage, suppressing inflammatory responses, etc. It is also noted that the mechanisms are not fully understood and the results remain controversial. However, they reflect more stability and less of a possibility of immune rejection following in vivo allogeneic administration than other cells.32 Studies have reported that exosomes from MSCs had the same immunomodulatory properties as the MSCs themselves.36
Epithelial cells and fibroblasts in patients with IPF are reported to accelerate senescence. Cellular senescence is the state of irreversible cell cycle arrest in response to cellular stresses. These cellular stresses can be a variety of things such as environmental exposures including radiation, drugs, infections, etc. Senescence can increase the secretion of exosomes and alter its contents such as proteins and miRNAs that induce cellular senescence. This exosome secretion may compensate for lysosome and autophagy dysfunction. In addition, aging increases cellular senescence. Evidence proposes that IPF may result from an accelerated aging lung. With that in mind, epithelial cells and fibroblasts in IPF are stated to accelerate senescence. Although the pathophysiological involvement of EVs in IPF has not been studied yet, the level of exosomal miR-21 is found to be higher in the serum of patients with IPF compared to healthy controls miR-21 plays an important role in the chronic, low-grade inflammation that characterizes aging. These claims show that exosomes may play an important role in the pathogenesis of IPF. If exosomes are the biomarkers of age and age-related diseases, such as IPF, then this identification could help lead to novel diagnostic classifications and therapies. Thus senescence associated exosomes may be a novel therapeutic strategy33.33
Another study viewed the microRNA (miRs) content of exosomes from sputum of patients with IPF. They found evident dysregulation of sputum exosomal miRNA levels between patients with IPF and healthy patients. They believe that this characterization of miRNA is a promising biomarker for diagnosis and disease severity. It showed that these sputum exosomal miRNAs are involved in the development of inflammatory diseases such as IPF. This study set up the backbone for further investigation of these biomarkers and their roles in IPF pathogenesis and thus opens new avenues for therapeutic approaches.37 Increasing evidence shows that miRNAs packaged in exosomes contribute to inflammation in many disease contexts. Therefore more studies need to be conducted to investigate the potential for exosomes to package the cellular mediators that drive inflammation in these diseases.35
MicroRNAs (miRs) are short, highly conserved small noncoding RNA molecules that play a huge role in regulating gene expression. A report identified these miR biomarkers associated with IPF in elderly patients from the expression pattern of miRs in exosomes from bronchoalveolar lavage fluid (BALF). By using a microarray, high-throughput quantitative detection of miR expression indicated upregulation of miR‑125b, miR‑128, miR‑21, miR‑100, miR‑140‑3p and miR‑374b and down regulation of let‑7d, miR‑103, miR‑26 and miR‑30a‑5p. They further examined miR-30a-5p and showed that the decreased expression of it in the BALF of patients with IPF may be a crucial factor in IPF progression. They also found a resulting effect of an increase in TAV3 expression which could contribute to IPF progression as well.38
Furthermore, exosomes are an extracellular vesicle that is tiny pouches released by a variety of cells that contain numerous messenger substances. These substances include proteins and nucleic acids. They are necessary for communication between cells and organs to help make sure that the substances reach new sites. One report states that is has been shown that there are increased levels of these vesicles in IPF patients and showed that reducing the number of vesicles decreased tissue scarring.39 Another report supported the idea that exosome therapeutics is an emerging field that provides an alternative to stem cell therapy. This report compared the benefits of exosomes to stem cells and found they have a number of measures where exosomes are superior. When comparing the benefits, they found that exosomes are non-cellular and are unable to form a tumor, they are readily available and easily administered. This was supported by their amniotic exosome treatment explained later.40
Extracellular vesicles (EV) have been implicated in lung cancer and asthma, acute lung injury, chronic obstructive pulmonary disease, acute lung transplant rejection, and sarcoidosis. However, it is unknown how these vesicles work in the pathogenesis of the diseases. The role of EVs in these diseases has sparked interest in their role as therapeutic targets or biomarkers.41 Furthermore, these extracellular vesicles have been proposed and used to be a therapeutic strategy for inflammatory lung diseases. Figure 5 represents the pathway in using these microvesicles starting from deriving them from mesenchymal stem cells.42
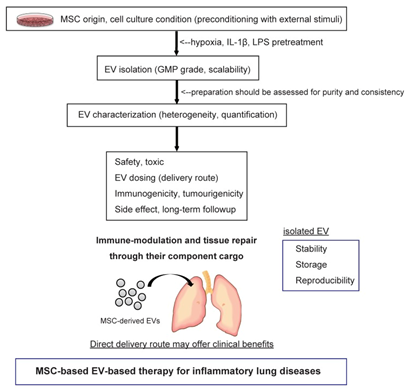
Figure 5 This represents the approach in obtaining extracellular vesicles from the mesenchymal stem cells and delivering them into the injured lungs.42
Preclinical studies with exosomes
Stem cell-derived exosomes or extracellular vesicles (EVs) are becoming a popular research topic for pulmonary and renal fibrosis, cutaneous wound healing, and myocardial infarction. In a report, human amnion epithelial cell-derived exosomes (hAEC Exo) were isolated and compared to human lung fibroblasts exosomes. This was to observe the exosomes protein and miRNA cargo. The study was comprised of hAEC Exo that were isolated from placentae of women undergoing elective caesarean section. The controls were exosomes from healthy human lung fibroblasts (HLF) to effectively compare these to the hAEC Exos under the same conditions. Next, the immune potency of hAEC Exo and HLF were assessed by in vitro. Lastly, mice were injected with bleomycin to construct a lung injury and then were randomly allocated with hAEC Exo or HLF Exo by intravenous injection. It was shown that hAEC Exo can produce anti-fibrotic, immunomodulatory and regenerative properties paving the way for overcoming the barriers of cell-based therapies by using exosomes.36
A report explained the characterization of extracellular vesicles and studied the role of extracellular vesicles-bound WNT (wingless/integrated) signaling in IPF. They isolated these extracellular vesicles from BAL fluid from experimental lung fibrosis as well as samples from IPF, non-IPF interstitial lung disease, non-ILF, and healthy volunteers from two independent cohorts. They found that EVs, especially exosomes, were increased in BALF from experimental lung fibrosis as well as from IPF patients. This showed that increased EVs function as carriers for signaling mediators and contribute to disease pathogenesis. This characterization of the vesicles can lead to novel therapeutic treatments for this disease.43
Another study demonstrated the efficacy of bone marrow-derived MSC exosomes (MEx) and their immunodulatory potential in a bleomycin IPF model. Exosomes were isolated by MSCs using iodixanol density gradient and characterized by electron microscopy and western blot. The results showed the administration of MEx produced a significant reduction in lung fibrosis and collagen content compared to the control group. This treatment also led to the reduction in apoptosis. Therefore, the MEx treatment showed a significant amelioration of pulmonary fibrosis.44 As stated before, one report explained their amniotic exosome treatment. They believe that their treatment is as effective, or better than, their producer human placental amniotic epithelial stem cells (hAECs) in preclinical models of fibrotic conditions. Their results displayed amniotic exosomes reversed the established lung inflammation and fibrosis in a mouse model of bleomycin-induced lung fibrosis. This result also showed the reduction of activated myofibroblasts and collagen deposition in the lungs. In addition, amniotic exosomes were shown to have a pro-regenerative effect where they caused an endogenous stem cell response in the lungs. They compared this result with hAECs and the amniotic exosomes had a significantly greater effect.40
Additionally, another study’s goal was to provide evidence that mesenchymal stem cells directly replace fibrosis with normal functioning lung cells by using model mice with IPF. Additionally, the study also aimed to examine the release of microvesicles from these stem cells and their therapeutic effects. This report stated the therapeutic effects of MV treatment is less than that of MSCs but that they have the ability to still contribute greatly to the reduction of collagen deposition and inflammation. To begin the study, they isolated hMSCs by using ExoQuick then exosomes were precipitated after incubation. The mice that received silica were injected with hMSCS or microvesicles (MV) released from hMSCs via the tail vein. This experiment showed the effect of MSC or MV treatment on lung wet/dry ratio for the mice that received the silica treatment. These effects showed that both hMSC and MV reduced the wet/dry ratio but only the hMSC treated mice showed meaningful results. Next, the mice were analyzed for the effects of hMSC or microvesicles (MV) treatment on BAL cells. These results included the decrease in the number of total BAL cells as well as neutrophils and lymphocytes for both the hMSC and microvesicle (MV) treated mice. It also showed a decrease in the percentage of foamy macrophages. The report states that this is directly correlated with extent of fibrosis and concentration of apoA1 in BAL fluid. In an additional test, the degree of fibrosis was also evaluated in the hMSCs or MV treatment using Masson’s trichome staining. This showed that the degree of inflammation significantly decreased at 12 weeks and 14 weeks after treatment. It also showed the amount of collagen I was decreased after both the hMSCs transplantation and microvesicle (MV) treatment which is shown in Figure 6. However, fibrosis was developed in the lung parenchyma of these silica treated mice. This study also detected if the hMSCs differentiated into functional lung I cells. They confirmed this by using a human nuclear antigen specific antibody. They achieved this by conducting an RT-PCR to examine if those hMSCs developed into functionally active lung cells or not. Total RNAs were extracted from the mice lung tissue and reverse transcribed. These results are shown in Figure 7. From this experiment, they found a marker of differentiated lung II cells which is known as human surfactant protein B. This was only found in the mice treated with hMSCs not microvesicle (MV) treatment. In conclusion of this study, it showed that microvesicles (MV) had a therapeutic effect on silica induced pulmonary fibrosis without transferring hMSCs. These microvesicles (MV) alone reduced the recruitment of inflammatory cells and collagen deposition.3
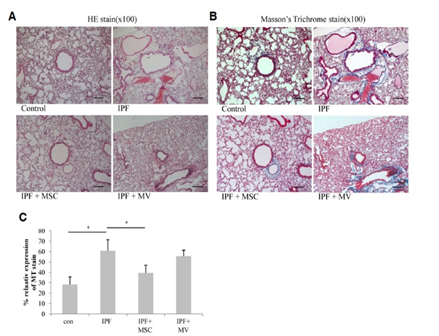
Figure 6 The effect of hMSCs and microvesicles was studied to treat silica induced pulmonary fibrosis mice. This figured shows how the transfer of these decreased the amount of collagen deposition in the lungs.3
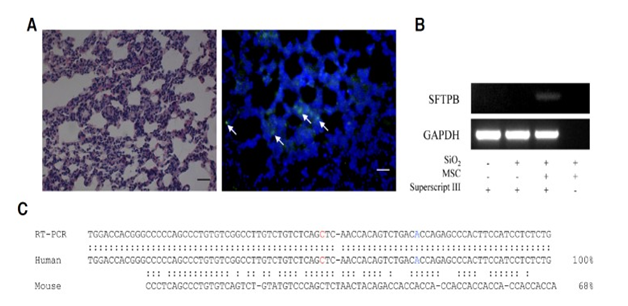
Figure 7 This figure represents the results of the detection of differentiated hMSCs in mouse lung tissues.
(a) Represents an image of H&E staining immuno fluorescent staining for human-specific nuclear antigen.
(b) Represents the detection of the surfactant protein B.
(c) Represents the comparison of the cDNA sequence in the mouse and the human surfactant protein B.
Lastly, a study displayed the therapeutic potential of intravenous delivery of MSC-derived exosomes in bleomycin-induced pulmonary fibrosis mice. Exosomes were isolated from adipose-derived MSCs from young male human and mouse donors. The mice were assessed and all treatment groups resulted in decreased pulmonary fibrosis compared to the bleomycin only controls. The other treatment groups consisted of bleomycin and whole-cell human or mouse MSCs and bleomycin and human or mouse MSC-derived exosomes. There was no difference in fibrotic endpoints between MSC and MSC-derived exosome treatments. Both the human MSC and human MSC-derived exosome treatments showed similar results in healing promotion. This data supported further investigation on the potential of MSC-derived exosomes for the treatment of lung diseases.45
In summary, MSCs and exosomes derived from MSCs have the potential to treat patients with IPF. Though there are no trials that have successfully cured patients, most of them have shown success in slowing down or reducing the symptoms such as fibrosis when administered in patients. Many reports have been identified that shed light on progress in this field. They have shown the potential and possibility of MSCs and exosomes to treat patients with this degenerative disease. IPF is from unknown causes that have to date no effective treatments. The two drugs that are approved are antifibrotic drugs such as Nintedanib and Pirfenidone. These drugs include slowing down the progression of the disease, but do not successfully treat or cure the condition. Stem cell therapy has become a great therapeutic option for patients with IPF. Stem cells have the ability to regenerate damaged tissue. With this characteristic, it is hopeful that stem cell therapy could regenerate and repair the damaged lung tissue in patients with idiopathic pulmonary fibrosis. In this review, the potential benefits and cases were all explained and reported through animal trials as well as clinical trials. It appears that stem cell therapy is a beneficial therapeutic option for patients with IPF. Exosomes are produced by various cell types that can be isolated from a variety of bodily fluids. These vesicles are found to have multiple functions that could treat idiopathic pulmonary fibrosis. They are known for their development of healthy cells, tissues, and organs and also to promote inflammation and immunosuppression. These exosomes can alter gene expression of target cells through their transfer of miRNAs causing in a diseased phenotype, which is why they are becoming appreciated for the pathogenesis of respiratory diseases. In addition, MSCs have a variety of beneficial properties such as homing, differentiation, and are shown to alleviate lung injury and fibrosis in BLM-induced models. These multipotent stem cells are able to differentiate into a number of different tissue lines. They also have the ability of anti-proliferative and anti-inflammatory effects. Although intense research efforts and clinical trials are being produced, IPF is gradually increasing worldwide. That is why increasing research has developed for new treatments that are safe and effective. More research and review is necessary. This disease places a great burden, financially and socially, on the patient and their families which calls for a desperate need for treatment. After reviewing the studies discussed above, the use of the MSCs and exosomes to treat IPF have provided successful stories. Furthermore, stem cells show promise for future treatment and even possibly a way to cure IPF as well as many other disorders.
None.
Authors declare that there is no conflict of interests.

©2019 Chase, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.