Journal of
eISSN: 2475-5540


Research Article Volume 6 Issue 1
Department of Biological Sciences, College of Science, Clemson University, USA
Correspondence: Dr. Vincent S. Gallicchio, Department of Biological Sciences, Clemson University, Clemson, SC 29636 USA
Received: January 20, 2020 | Published: January 29, 2020
Citation: Crisologo M, Gallicchio VS. Evaluation of stem cell therapies for amyotrophic lateral sclerosis. J Stem Cell Res Ther. 2020;6(1):11?21. DOI: 10.15406/jsrt.2020.06.00136
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disorder for which treatment consists mainly of palliative care. Two drugs are currently widely available in the US for treatment, Riluzole and Edaravone, which can mildly attenuate motor decline and slightly prolong survival. Stem cells are currently being explored as treatment possibilities because of their ability to differentiate to healthy motor neurons and astrocytes especially, which are thought to be a major source of the neuronal damage by engaging in a positive feedback loop of neuroinflammation. Various stem cell types are also known to secrete neurotrophic factors which can promote healthy astrocyte function and mediate axonal regeneration and repair. This leads to two broad classifications of stem cell therapies: Replacement and Non-replacement. Replacement therapies tend to use neural stem cells to supplant the host’s diseased astrocytes and create a healthy environment. Motor replacement is less feasible due to the distance they need to grow to reach the neuromuscular junction and difficulties of integration. Non-replacement therapies tend to use bone marrow mesenchymal stromal cells and tend to focus on immunomodulation to reduce damage to the motor neurons. Results from animal trials and phase I/II clinical trials show that both types of treatment using stem cells such as neural stem cells, bone marrow mesenchymal stem cells, dental pulp, and adipose derived stem cells can reduce neuroinflammation and motor neuron degradation, attenuate motor decline, and in many cases prolong survival. Future studies should look to the application of combined replacement and non-replacement strategies using both neural stem cells and mesenchymal stem cells to achieve an even greater level of neuroprotection.
Keywords: ALS, neural stem cells, embryonic stem cells, mesenchymal stromal cells, neuroinflammation
ALS, Amyotrophic Lateral Sclerosis; NTF, Neurotrophic Factor; ESC, Embryonic stem cell; NSC, Neural stem cell NPC, Neural progenitor cell; hNSC, human neural stem cells; hESC, human embryonic stem cells; MSC, Mesenchymal stromal cell; BM-MSC, Bone marrow derived mesenchymal stromal cell; NMJ, Neuromuscular junction; ALSFRS-R, ALS functional rating scale-revised; FVC, Forced Vital Capacity; HSPC, Hematopoietic stem and progenitor cells; BBB, Blood brain barrier; BSCB, Blood spinal cord barrier; C9ORF72, Chromosome 9 Open Reading Frame 72; SOD1, Superoxide Dismutase 1 gene; FUS, Fused in Sarcoma gene; TARDBP, Tar DNA binding protein gene; PGP, P-glycoprotein; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; TDP-43, Tar DNA binding protein 43; STMN2, Stathmin 2; JNK, c-Jun N-terminal kinases; ATG4B, Autophagy Related 4B; miRNA, micro RNA; RNA, ribonucleic acid; DNA, deoxyribonucleic acid; GABA, γ-aminobutyric acid; HSF1, Heat shock factor 1; HBSS, Hank’s balanced salt solution; MN, motor neuron; GLAST, Glutamate aspartate transporter; GFAP, Glial Fibrillary acidic protein; ESC-AS, embryonic stem cell derived astrocyte; DAT, days after transplantation; DMEM, Dulbecco’s modified Eagle media; CNS, Central nervous system; GDNF, glial derived neurotrophic factor; BBB scale, Basso, Beattie, and Bresnahan motor scale; FVC, Forced vital capacity; ALSFRS-R, ALS functional rating scale- revised; CSF, Cerebrospinal fluid; CM, Conditioned media; CHAT, Choline acetyltransferase; HSPC, Hematopoietic stem progenitor cell; PBMC, peripheral blood mononuclear cell; IFN-γ, Interferon gamma; TNF- , Tumor necrosis factor Alpha; IL-6, Interleukin-6; IL-10, Interleukin-10; CD4,8,25, Cluster of Differentiation 4,8,25; ADRC, Adipose derived regenerative cell; AALS, Appel ALS Rating Scale; TGF-β, Transforming growth factor beta; MCP-1, Monocyte chemoattractant protein 1; ASC, adipose stem cell; sALS, sporadic ALS; fALS, familial ALS; C3/4, complement component 3/4; NGF, Nerve growth factor; BDNF, Brain derived neurotrophic factor
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disorder that is generally fatal within 2-5 years.1 It is characterized by degeneration of the motor pathways to both the lower and upper limbs, followed by severe muscle atrophy.2 Cause of death is primarily respiratory failure, as the degeneration of the motor neurons affecting respiratory muscles progresses.2 A fully unified mechanism for the cause of the disease and its progression is yet to be determined, however an overview of the various contributing factors is presented in the mechanism section of this paper. Current standard of care consists mainly of palliative care and symptom management.3 There are two drugs available to treat ALS: Riluzole, a glutamate uptake enhancer, and Edaravone, a free radical scavenger. Riluzole has not been shown to affect disease progression but extends survival by about 3 months during the final stage of ALS.4,5 Edaravone can slow the decline in motor function in early stages of ALS in patients with mild symptoms.6 With only two treatments currently available, new methods are imperative in making ALS prognosis more promising. Stem cells have been explored as a novel treatment method because of their ability to differentiate into healthy cells, thereby supporting the diseased nervous system. Stem cells also exhibit other properties, such as immunomodulation and secretion of neurotrophic factors (NTFs) that can exert a neuroprotective effect. This review aims to summarize the information on the mechanism of neurodegeneration and place it in the context of stem cell-based therapies to combat ALS.
Pathophysiologic mechanism
The full mechanism of ALS is still yet to be completely determined. How the disease begins and how it causes neuronal death/degradation are some of the questions that require the most attention in terms of what to research. Other mechanisms are much more understood. One such mechanism is the role of the glial cells and the immune response to ALS. Microglial cells, and astrocytes in particular, that have known ALS causing mutations (C9ORF72, SOD1, FUS, TARDBP, etc.) have been shown to be abnormally sensitive to the neural degradation and release greater amounts of inflammatory cytokines.7‒9 The increased number of inflammatory cytokines leads to further MN degradation, which in turn, provokes further response from the astrocytes in a positive feedback loop.7‒9 Astrocytes and Pericytes, another type of Glial cell, have also been implicated in the maintenance of the Blood Brain Barrier, and their dysfunction in ALS phenotypes has been shown to decrease the integrity of both the BBB and BSCB by reduced expression of certain endothelial markers present on the membrane and upregulation of PGP, a BBB/BSCB transporter.9‒11 This increased permeability allows for greater penetration of damaging particles into the neuron’s environment. The extracellular environment of the motor neurons is also regulated by astrocytes.8,12 They are responsible for the regulation of ion levels in the areas between neurons.8,12 ALS displays an altered extracellular environment, containing greater amounts of most ions, most notably Ca+2 and glutamate.9,12 Astrocytes specifically have been implicated in the decreased uptake and increased release of glutamate.9‒11 ALS motor neurons have been shown to be hypersensitive to metabolites, especially glutamate.12,13 Motor neurons bearing hallmark ALS mutations overexpress certain glutamate receptors (AMPA, Kainate receptors, metabotropic glutamate receptors) in the synapses of the neurons increasing their sensitivity to toxic levels.12 Overexpression of voltage gated Ca+2 channels has also been observed.12 Another mechanism of pathology acting in the astrocytes is a decreased autophagy response due to misfolded protein aggregates.8 Like other neurodegenerative diseases, protein inclusions are a hallmark of ALS pathology, and like in other neurodegenerative diseases the exact mechanism of toxicity of these inclusions is not entirely known. One protein inclusion, TDP-43 is found in ~95% of all ALS cases, and regulates the transcription of almost 900 transcripts.14 STMN2 regulates axon regeneration after damage and its transcripts were shown to be particularly sensitive to loss of TDP-43 function. STMN2 expression is greatly reduced in ALS patients, and inhibition of JNK, a protein that marks STMN2 for degradation by phosphorylation, rescued the reduced expression.14 TDP-43 is a regulator of ATG4B, an autophagy enzyme, and dysfunction of TDP-43 leads to loss-of-function alternative splicing in ATG4B.15 TDP-43 aggregates induce expression of p53, upregulating apoptosis.16 TDP-43, along with C9ORF72 products and FUS products also play a role in RNA metabolism.17-19 Loss of function in these genes is correlated with downregulation of miRNAs that regulate pathways including response to oxidative stress, senescence, GABA synthesis, release, reuptake, and degradation.17,18,20 Loss of function in C9ORF72 produces dipeptide repeats that inhibit the nonsense-mediated decay pathway and induce transcription of heat shock proteins and HSF1, leading to neurotoxicity.18,21,22 Mutant FUS aggregations have been shown to localize in the cytoplasm of neurons as well as different synaptic compartments based on maturity of the disease.23 Less mature cells show localization in the postsynaptic area, and more mature synapses show almost exclusively presynaptic localization.23 Scekic-Zahirovic et al. show that up-regulating mutations in FUS are linked to muscle denervation, defective oligodendrocytes, and abnormal myelination.24 Mutations in FUS also affect its ability to regulate DNA damage repair.25 Localization outside the nucleus stops FUS from performing its normal function of recruitment and activation of DNA Ligase IIIa to sites of DNA damage.25 Mutant FUS also increases the sensitivity of astrocytes to pro-inflammatory factors, feeding the positive feedback loop.26 SOD1 mutant proteins have been shown to block protein channels in the outer mitochondrial membrane, reducing anion transport and thus mitochondrial function.27 Motor neurons will also take up SOD1 aggregates in the environment, allowing the inclusions to induce apoptosis in multiple neurons and spread throughout the nervous system.28 Possible environmental factors that affect generation of SOD1 aggregates could be low O2 tension in the cellular environment, increasing protein disorder and the likelihood of an inclusion forming.29 The free radical scavenging function of SOD1 is also compromised in mutant forms, leading to more free radical induced damage. Interestingly, a mouse model showed that if the SOD1 mutation was excluded from astrocytes and only expressed in motor neurons, no ALS symptoms presented.8 This could compound with the reduced integrity of the BSCB and reduced DNA repair function of FUS for even greater destructive potential.
Treatments involving stem cells can be broadly grouped into two different categories, replacement and non-replacement therapies. Replacement therapies generally use neural stem cells, neural progenitor cells, or pluripotent stem cells to differentiate healthy motor neurons or glial cells. The goal of these treatments is to replace the diseased tissues with healthy ones. Non-replacement therapies generally use stem cells that won’t differentiate to a neural phenotype, but support the CNS by secretion of neurotrophic factors, promoting a healthier neuro-environment, and most importantly by modulating the immune response to nerve degradation and reducing neuroinflammation.
Replacement therapies
Complete cell replacement therapy has yet to be shown.30,31 Replacement motor neuron’s are not able to extend all the way to the neuromuscular junction to innervate muscles.31 The introduction of healthy motor neurons has still been suggested to have a therapeutic effect by helping to innervate interneurons in the spinal cord.32,33 Xu et al. injected SOD1 mice by lumbar puncture with human Neural Stem Cells (hNSCs) isolated from an aborted 8-week human fetus and found that the cells were able to differentiate, engraft, and survive in the diseased environment.32,33 The engrafted hNSC derived motor neurons were found to form connections with host motor neurons, but not innervate the neuromuscular junctions (NMJs).33 In addition to strengthening connections within the spinal cord, Xu et al. suggest the hNSC derived motor neurons were exerting a protective effect on host neurons through delivery of neurotrophic factors (NTFs), which have previously been shown to exert a protective effect in an excitotoxic environment.33,34 NTFs are known to exert neuroprotective effects, improve motor performance, and increase lifespan in mouse models of ALS, likely by supporting and promoting healthy glial cell activation.30
In an animal trial of the effects hNSCs, 15 transgenic rats with mutated SOD1 were given injections of hNSCs, 22 SOD1 rats were used as controls, and another 15 were treated with a vehicle HBSS.35 Stem cells were sourced from an established cell line from Cell Factory and Biobank of Santa Maria Hospital in Terni, Italy. Transplantation occurred when symptoms in rats began to manifest, measured by decreased limb control and rotarod failure. Cells were injected into the ventral horn of the lumbar region at 4 different sites, with a total of 400,000 cells being delivered to each animal. Progression of the disease was monitored by rotarod tests, a motor scoring test based on the level of paralysis in the limbs, and body weight. 5 rats were randomly selected for euthanizing for immunohistochemical evaluation at 15 days after transplantation, 40 days after, and at end stage (60 days). Data on cell survival, migration, proliferation, and differentiation are summarized in Tables 1 and 2.35
Survival |
Migration (cm) |
Proliferation |
|
15DAT |
53±32% |
1.69±0.47 |
9±3% |
40DAT |
78±13% |
1.02± 0.39 |
14±4% |
End |
54±19% |
3.77±0.63 |
15±2% |
Table 1 Proportion of surviving stem cells, migration distance, and proliferation rates at various time points after transplantation
Proportions of surviving cells remained high throughout the experiment and were even seen to be proliferating at low levels, generating new stem cells in vivo (Table 1). The cells were also able to disperse along the spinal cord and migrate in vivo (Table 1). Cells mainly differentiated to an astroglia type, but also differentiated into motor neurons and oligodendrocyte precursors (Table 2). Rats receiving the transplants were observed to have a significantly longer survival time (23 days, p=0.018) and also exhibited a slower progression of symptoms in all categories measured (rotarod, motor scoring, and body weight, p≤0.02 compared to vehicle). Scores in these categories were decreased for about 10 days after the surgery in both the experimental and vehicle groups, indicating this is likely due to recovery from the operation. Immunohistochemical analysis of the spinal cords also revealed that there were significantly more intact motor neurons (MNs) in the hNSC group, with the highest density found closest to the injection sites (p=.05 compared to vehicle). SOD1 inclusions were also found to be reduced at 40DAT. hNSC treated groups also showed reduced astro-/microgliosis at 15 and 40 DAT. This shows the viability of hNSCs in vivo and that they are able to safely and effectively incorporate into a diseased host environment. High levels of cells are able to survive to for at least 2 months, migrate along the host spine, differentiate, and are seen to proliferate at low levels to produce new stem cells, while refraining from becoming tumorigenic. hNSCs were also seen to significantly slow the course of the disease, extend survival, and reduce neuroinflammation by decreasing astro/microglisosis. Reduced neuroinflammation and SOD1 aggregates in the environment, combined with other functions of healthy astrocytes, such as glutamate uptake, are likely the cause for the decreased neuronal degradation as well.35
Nestin |
GFAP |
B-tubulin |
PDGFRa |
|
15DAT |
26.9 ± 7.3% |
25 ± 4.7% |
16.1 ± 2.2% |
9.6 ± 3.7% |
40DAT |
21.7 ± 7.7% |
20.5 ± 7.7% |
10.7 ± 5.4% |
17.6 ± 2.9% |
End |
Sporadic |
34.4 ± 3% |
4.5 ± 2% |
9.4 ± 1.6% |
Table 2 Proportion of cells expressing markers indicating differentiation to different phenotypes
Izrael et al.36 differentiated astrocyte precursors from human embryonic stem cells (hESCs) in vitro to develop astrocyte progenitor banks that could be frozen and stored for later use. Precursor cells expressed very low amounts of pluripotency markers (<0.02% of cells) and high levels of astrocyte marker CD44 (~90%). The cell banks were frozen for 2 months before being thawed, expanded, and differentiated toward committed astrocytes in a period of about 4 weeks. During this time levels of other astrocytic markers (GLAST, GFAP, etc.) increased while pluripotency markers remained low. ESC derived astrocyte (ESC-AS) glutamate uptake was compared to astrocytes taken from a healthy adult spinal cord and found to exhibit similar levels of glutamate uptake at all time points. ESC-AS were also found to exhibit oxidative stress protection (rat spinal MNs, p<.01 compared to untreated control), axonal growth stimulation (rat cortical neurons, p<.01 compared to untreated control), and NTF secretion. To evaluate their effects in vivo, SOD1 transgenic mice and SOD1 transgenic rats were transplanted with ESC-AS. In the transgenic mice, 2x106 cells were injected into the CSF through the cisterna magna once at 67 days old (n=14), twice on days 67 and 97 (n=13), or with a vehicle treatment (n=10). Mice were then evaluated on rotarod, body weight, and neurological scoring based on level of limb function. Time to onset was determined by loss of 3% maximal body weight. Baselines were taken for the week prior to the operation. The double transplantation was found to significantly delay disease onset and improve rotarod performance. In the rat model, a total of 6x106 cells were delivered intrathecally by lumbar puncture over two injections at 50 and 70 days old (n=7). A vehicle group was injected in the same way with only DMEM (n=7). Rats were evaluated with the same tests as the mice, with the inclusion of a grip strength test. Disease onset was significantly delayed (p=.0001), and scores on rotarod, neurological testing, and grip strength also showed a significantly reduced progression (p<.001). Treatment trended toward increasing survival, however this effect was not significant (p=.077). No tumorigenic activity was detected and blood chemistry between the two groups was identical. ESC-AS were found to be present on all levels in the CNS, with the highest densities being found near injection sites. No correlation between cell transplantation and increased survival time is interesting, however this is likely due in large part to small sample sizes, especially considering survival time was trending toward statistical significance.36
One of the many mechanisms for neuroprotection coming from transplanted stem cells is the release of neurotrophic factors that can promote axonal growth/repair and normal glial cell function. Genetically engineering human neural progenitor cells (hNPCs) to differentiate into astrocytes that secrete greater amounts of glial cell line-derived neurotrophic factor (GDNF) has been explored as a therapeutic route. hNPCs were engineered to secrete GDNF and transplanted into the cortex of SOD1 rats. Cells were injected into 20 different sites of the cortex for a total of 400,000 cells per animal. Locomotion was evaluated by on the BBB scale, a limb function test used to evaluate the level of paralysis in any specific limb. Animals were evaluated once or twice weekly using the scale starting at 100 days old and continuing until disease endpoint, which was 20-30% body weight lost and a score of less than 5 in any limb. Transplants were detectable at endpoint in 69% of subjects (9/13) and differentiated mainly into GFAP+ astrocytes, and secondarily into nestin expressing cells (60% and 20% respectively). Widespread GDNF was detectable in the motor cortex as well. Treatment with GDNF hNPCs was associated with a delay in disease onset, longer survival, and improved motor function in both hind and forelimbs (p<.05 compared with noninjected controls and WT hNPCs). This effect was determined to be due to the increased GDNF secretion, as injection with WT hNPCs did not produce similar effects. To compare the effects of the treatment at different timepoints, random rats of each group were euthanized at day 165 and compared. GDNF hNPCs slowed the degradation of large (>500um2) corticospinal MNs compared to controls in both endpoint and time point (p<.05), however small (>300um2) did not show any significant slowing at endpoint or at the earlier time. In the spinal cord, the GDNF hNPC group showed significantly increased large spinal MNs, total spinal MNs, and increased average cell size at the earlier timepoint compared to endpoint rats in both groups (p<.05), but only a significant increase in large spinal MNs and average cell size compared to non-injected cells at same timepoint (p<.05). The difference in total cells had a p value of .08. No cell migration nor GDNF was detected in the spinal cord, suggesting that the protection was due to the support of the cortical neurons. Thomsen et al. also investigated whether engineered hNPCs could be safely delivered to the cortices of larger animals, delivering 500 000 cells to 12 injection sites in the cortex of Cynomolgus Macaque. No adverse effects were observed 30 days post operation in the primate and immunohistochemical analysis afterward revealed high amounts of cell survival and GDNF secretion and uptake in the cortex.37
A phase I clinical trial transplanted NSCs to ALS patients to primarily evaluate the safety, and secondarily to evaluate the efficacy of the treatment. 18 total patients, median age of 48, having been diagnosed with ALS for at least 6 months were given 3 or 4 injections of 750,000 cells/injection. Patient FVC, ALSFRS-R, Medical Research Council Scale, and Ashworth Spasticity Scale baseline was taken in the 3 months leading up to the procedure. MRC evaluates muscle function in different upper and lower limb muscle groups, while the Ashworth scale measures increases in muscle tone. Median follow up was 24 months. Adverse effects include 1 case of transitory respiratory failure, starting 1 day after surgery and was supported with noninvasive ventilation and resolved within 24 hours. No patient displayed immediate post-operative respiratory difficulties. 1 patient developed iatrogenic diabetes 1 week after operation and put on long term insulin. The most common side effect of the operation was mild pain localized at the surgical site, rated at II-III on the WHO scale, treated with narcotic and non-narcotic analgesics, and generally resolving within 18 days. 1 patient’s pain did not resolve until 30 days later, and 1 did not resolve until 120 days later. Pain was most closely related to injections that required a laminectomy, rather than the number of injections or the number of cells. No tumor formation was observed, and treatment was not associated with an acceleration of ALS progression. In regard to the secondary objective of the study, comparing the rates of change of ALSFRS-R scores from the 3 month lead in to the rates of change from the follow-up show that treatment was associated with a transitory slowing of disease progression for the first 4 months post operation (p=.0136). 4 patients showed an improvement of their upper limb function, increasing their upper limb ALSFRS-R score by 1 point, 2 showed improvement of MRC in proximal lower limb muscles, 1 showed improved MRC score of proximal upper limb muscles, and 1 patient displayed a particularly rapid decline of ALSFRS-R score prior to transplantation that attenuated for 6 months. Other patients had their decline lessened for between 3 and 6 months, with the most mild phenotype experiencing the effects for around 12 months. Improved scores lasted anywhere between 2 and 6 months after surgery. NSCs used were also treated with patient CSF in vitro and compared with NSCs treated with saline and healthy CSF to determine if there was a different pattern of differentiation. No significant difference was found, with about 60% differentiating into GFAP positive cells (astrocytes), 10% into β-tubulin III (MNs), and 30% GalC cells. NSCs were sourced from donor miscarried fetus cells. Overall this study demonstrates the safety of transplanting NSCs into a patient with ALS. No adverse effects post operation were related to the cells, but rather to the procedure itself, and were all easily managed, with most resolving in about 2 weeks. Stem cells are also seen to differentiate normally in the diseased host environment in vitro, and no tumor formation being observed also support stem cell transplantation being a safe therapy. Moreover, transplantation was associated with a transitory slowing of progression and in some cases improvement of muscle function, evaluated by ALSFRS-R and MRC, however FVC score showed no similar improvement or decline in progression. Treatment appeared to be more effective in the lower limbs than the upper limbs overall, possibly indicating a differential effect based on location of cell delivery. This could be indicative of different effects exerted by the stem cells in the cervical area compared to the lumbar, it could possibly be because of a differential mechanism occurring in cervical nerves, or perhaps cervical nerves are less sensitive to the effects of stem cells. Dosages in this study are also likely lower than the therapeutic level, as its main goal was to assess safety of the procedure. However, conclusions of efficacy are difficult to determine, mainly due to the small sample size presented, as the authors note.38
Non-replacement therapies
Non-replacement stem cell therapies are characterized by their use of stem cells, not for the purpose of differentiating into a neuronal phenotype, but rather for other therapeutic properties, most commonly the reduction of neuroinflammation and the release of NTFs. The neuroinflammation feedback loop is one of the primary drivers of MN death in ALS, and many types of stem cells have been shown to be immunomodulatory and able to reduce this inflammation. NTFs have also been shown to greatly impact the survival of ALS motor neurons, preventing cell death, promoting healthy microglial function, and preserving the neuromuscular junctions. Non-replacement therapies offer several other advantages over replacement therapies, such as greater flexibility in source (Bone marrow, adipose, dental pulp, and even autologous samples), and delivery method (systemic delivery, rather than intrathecal). By avoiding the use of NSCs or ESCs, non-replacement methods are also able to skirt any ethical issues that may arise.
Wang et al. treated pre-symptomatic and symptomatic SOD1 mice with conditioned media from dental pulp stem cells, which have been shown to produce NTFs such as neurotrphin-3, GDNF, brain-derived neurotrophic factor, and nerve growth factor.39 Mouse NMJ’s and motor neurons were quantified and gastrocnemius muscles were weighed to quantify the effects of the treatment at the end stage of the disease, determined as the point in time the mouse could not right itself for 30 seconds after being placed on its side. Pre-symptomatic treatment significantly reduced NMJ denervation, muscle atrophy, and motor neuron loss, but did not affect astrogliosis. Post-symptomatic treatment was found to significantly extend the overall lifespan of mice by about 10 days. The mechanism and site (at the NMJ, at neuron soma, or both) of the conditioned media remains unclear. This study shows that the secretome of dental pulp stem cells is effective at slowing denervation and neuronal decay but does not significantly affect glial cell activity. While using media conditioned with the secretome of NTF secreting cells is effective, the longer duration of the disease in humans could present problems for long term treatment. More effective solutions would likely come from engrafted cells continually secreting these protective NTFs, which is also why determining the site of action is so important in this particular case.39
In a study on the different delivery routes of bone marrow mesenchymal stromal cells (MSCs), SOD1 mice were given repeated intramuscular, intrathecal, and a combination of both.40 Human MSCs were sourced from the Prague based company Bioinovia Ltd. SOD1 mice were split into 6 different groups; the first consisted of mice given hMSCs intrathecally and intramuscularly (SC+M, n=12). The second was mice only given cells intrathecally (SC, n=9). The third was the intramuscular group (M, n=8). The fourth group was given only conditioned media from the hMSCs (CM, n=8). 12 mice were given a vehicle treatment intrathecally and intramuscularly, and finally 10 mice were of wild type, lacking the SOD1G93A mutation. An additional 6 SOD1 mice and 6 WT mice were used to determine hMSC survival at 7,14, and 28 days after transplantation. Transplantation occurred at symptom onset in each mouse, defined as the second straight week of weight loss. Transplantation was repeated 14 and 28 days after the initial operation. Intrathecal treatments were given through lumbar puncture, 5x105 cells or 100μL of CM. Cells delivered intramuscularly were split between three injections into the quadriceps femoris for a total of 2x105 cells. Mice were monitored and disease progression measured by body weight, BBB scale, rotarod, and grip strength. Tests were performed weekly beginning 2 weeks prior to transplantation. Starting at week 23, the SC and SC+M groups displayed significantly greater BBB scores compared to the vehicle treatment, the rate of disease progression was slowed in the M and CM groups in week 23-28, excluding week 26, and all groups showed improved rotarod performance at week 23 compared to the vehicle, except for the CM mice. The researchers noted that the overall physical condition of all treated mice assessed by rotarod was most like the WT mice until week 25, with the M group remaining similar for 1 extra week. There were no significant differences in the body weight and grip strength tests. SC+M, SC, and groups prolonged survival compared to CM and vehicle treatments (p<.001, p<.01, and p<.05 respectively). Analysis of CHAT levels showed that SC+M mice had reduced motor neuron loss compared to the vehicle (p<.05). This was confirmed through TUNEL staining, which showed decreased levels of neuron death. When investigating transplant survival rates, it was found that cells survived for 2-3 weeks in the spine, and no longer than 1 week in the intramuscular sites. When investigating possible causes for the therapeutic benefits, it was found that the SC+M treatment downregulated various transcripts related to necroptosis back to WT levels, and also downregulated proteins related to the necroptosis, autophagy, and apoptosis. In the combination treatment, NMJs were seen to be preserved, however this was not the case in the muscular only, or intrathecal only treatment, indicating that NMJ preservation was due to some synergistic effect. Combination therapy also reduced the levels of proinflammatory factors NF-kB and TNF-a. The low effect of the intramuscular group is likely compounded by the very short survival time of the cells, dampening any effect they would have at the NMJ. When applied in combination, however, the spinal MSCs could be secreting some protein into the bloodstream that could support the survival of cells at the muscular sites, allowing them to take effect longer. It could also suggest that the site of retraction is not at the NMJ itself, but rather the MN is retracting more at the cell body. Overall, this study demonstrates that MSCs are capable of a therapeutic effect on ALS cells by reducing neuroinflammation and preserving NMJs, especially when applied both intrathecally and intramuscularly.40
Another study further investigated the effects of human bone marrow MSCs when delivered systemically to SOD1 mice. Intravenous injections of 5x104, 5x105, and 1x106 cells were given to SOD1 mice at 13 weeks old which were euthanized 4 weeks later to evaluate spinal capillary and myelin integrity. It was found that there was significant restoration of capillary structure to the wild type form, improvement of the basement membrane, enhanced myelin coherence, and stabilized capillary density of mice receiving the highest dose of MSCs. Decreased BSCB integrity is one of the many symptoms of ALS that can feed into the neuroinflammation loop caused by damaged neurons. The study suggests that systemic delivery of BM-MSCs can be used to improve BSCB integrity/health, which could go a long way for protecting the vulnerable and damaged nerve environment from becoming damaged further.41
Some studies aim to use autologous stem cell populations as therapies. Granulocyte colony-stimulating factor (G-CSF) is a known hematopoietic stem cell mobilization, production, and differentiation factor that can be used to activate hematopoietic stem cells in hopes of having a therapeutic effect.42 To determine the effects of G-CSF administration on an ALS phenotype, 36 patients (mean age 60 years) were given subcutaneous injections of G-CSF over 5 days every other week, or every other day continuously for around 17 months. Dosages were determined on an individual basis, ranging from 900-21600 mg/month. Blood was drawn and blood count and cytokines were taken prior to treatment to determine baseline and then repeated once a month during treatment. Pro/anti inflammatory factor levels were taken at baseline, 3 months, and 6 months during treatment by multiplex electrochemoluminescence. The progression of ALS in each patient was monitored by ALSFRS-R. The subcutaneous injections were tolerated well, with mild to moderate bone pain and leukocytosis being the most common effects. 1 patient reported light headedness and heat flashes, with a 15 min period of dyspnea. Treatment was discontinued for fear of an allergic reaction, however no antibodies for G-CSF were found. The 5-day treatment showed sustained increases in the mobilization of hematopoietic stem and progenitor cells (HSPCs; p<.0002) and also was correlated with longer survival times (p=.01). Pro inflammatory cytokine levels were generally reduced, while anti-inflammatory levels were increased, even after 6 months in the 5-day treatment. Interestingly, the group receiving injections every other day showed no similar improvements. This could suggest the every other day group never reached a high enough level of G-CSF in the blood to have a therapeutic effect. The mechanism of action is not entirely understood; however, it is likely that the mobilized HPSCs translocate to the CNS and act as immunomodulators, reducing inflammation, or secrete NTFs that act on the CNS, or possibly both. Neural stem cells also have a receptor for G-CSF and therefore could be being activated as well.43 What is clear, is that mobilization of HPSCs was strongly associated with increased survival and reduced inflammatory cytokine levels in ALS patients.
Autologous bone marrow MSCs have also been explored as a therapeutic avenue.44 In an in vitro study on the immunomodulator properties of MSCs, peripheral blood mononuclear cells (PBMCs) were treated with MSCs from 14 ALS patients and 14 healthy controls. All ALS patients were ambulatory, early stage, and none needed ventilation or were malnourished. MSCs were isolated from the nucleated cell fraction of bone marrow samples (1x107 cells total) and then frozen in DMEM and DMSO with liquid nitrogen. Cells were later thawed, and half were cultured with inflammatory cytokines IFN-g, TNF-a, and IL-6 for 24 hours. In the end there were four total groups: ALS MSCs (ALS), healthy MSCs (HC), ALS MSCs treated with cytokines (ALSc), and healthy MSCs treated with cytokines (HCc). Cytokine groups were washed an extra time to remove added cytokines. Each of these groups were then cultured with 1x106 PBMCs in ratios between 20:1 and 320:1. All MSC groups were characterized by flow cytometry and no significant differences were found, indicating all the MSC populations were made up of similar cells. All the cells were observed to be expressing immunomodulatory genes similarly as well. Analysis of the cytokine levels of the PBMC+treatment culture revealed that all groups increased the amount of anti-inflammatory IL-10 being produced and decreased to pro-inflammatory TNF-a, however the ALS group decreased TNF-a levels more than the HC group (p<.01). No difference in TNF-a reduction was found between ALSc and HCc groups. ALS and HC groups also decreased the number of activated t-lymphocytes, evaluated as the number of CD4 and CD8 cells co-expressing CD25. The ALSc group reduced the amount of activated CD8 cells by a significantly smaller amount than the HCc group (p<.05). All groups reduced neurotoxic factors in ALS such as the number of T-BET+ T helper 1 lymphocytes, and also the amount of IFN-g. Groups also increased the number of neuroprotective factors like GATA-3+ T-helper 2 cells and IL-10. Every group except ALSc increased FOXP3+ Treg, a neuroprotective anti-inflammatory agent. Cytokine groups reduced the amount of neurotoxic RORγt+ Th17 lymphocytes, however no similar reduction was detected in the other groups.44 Taken together, this study provides evidence that both healthy MSCs and MSCs derived from ALS patients are able to modulate the immune response of the body, hopefully reducing the amount of neuroinflammation. TNF-a has been linked to neuroinflammation in ALS and its reduction here is very promising for alleviating inflammation in vivo.45 Effects of this study did seem to be modulated by pre-treatment with cytokines, especially in cells derived from ALS patients. This could suggest that autologous cells delivered to the highly inflamed CNS of ALS would be slightly less effective than cells derived from healthy donors, however small sample sizes and the in vitro model make the in vivo effects difficult to determine (Figure 1).
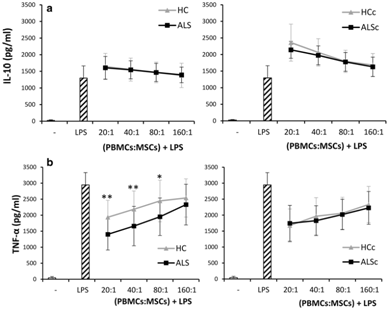
Figure 1 Comparison of cytokine levels between ALS patients treated with ALS MSCs (ALS) or healthy MSCs (HC) in PBMC culture. All cultures stimulated by LPS. Cytokine levels measured by ELISA. Asterisks indicate significant difference (* = p<.05, ** = p<.01).
Adipose derived regenerative cells (ADRCs) were isolated from patient adipose tissue and injected intraspinally (16,000 total ADRCs) and later twice intrathecally (5.6x107 total cells).46 4 patients were involved, all expressing upper limb dominant forms of ALS with a mean disease duration of 19 months. Patients had their baseline motor function, CSF cytokines, and ALSFRS-R score evaluated during a 3 month lead in to the transplantation and then every 3 months thereafter. Adverse effects related to the procedure include pain at the site of liposuction which resolved within 2 days, and superficial sensation impairment near the insertion site of the cannula. Moderate pain associated with laminectomy was reported and managed with analgesics for 1 week after which it disappeared. 1 patient reported headache following the intrathecal injection without signs of meningeal irritation. Another patient reported intubation related speech impairment which resolved in 10 days. 1 patient died 29 months following transplantation, however this was related to a gastronomy operation two days prior. Two patients reported increased walking capacity immediately following the procedure. In a two-minute walking test one of them was found to show an increase from 100.5m with a fatigability of 8 to 128.0m with a fatigability of 5 seven days after the procedure. The other was unable to perform the 2-minute walking test immediately following the operation due to pain and showed no improvement at 3 months post operation. According to the two subjects, the effects persisted for 4 weeks following the intraspinal injection and did not occur following either intrathecal injection. The rate of decline in the 2-minute walking test was somewhat reduced among all patients at 3 months. In the patients CSF, after 24 hours pro inflammatory levels were seen to be reduced, while anti inflammatory factors were increased at all timepoints CSF was taken. This study provides limited evidence for the efficacy of ADRCs delivered intraspinally and intrathecally for treatment of ALS. The most promising evidence comes from CSF analysis of patients, where treatment seemed to alleviate inflammation, however translating this data into tangible effects on the motor capacity of the patients was difficult. A major limiting factor of this study is the small sample size.
Repeated intrathecal injections of autologously derived bone marrow MSCs were given to assess their safety and efficacy in ALS patients.47 Two injections were delivered 26 days apart of 1x106 cells/kg body mass. Patients were also given a Riluzole regimen of 100mg/day. Baseline ALSFRS-R, FVC, Appel ALS Rating Scale (AALS), and cytokines from the CSF were taken during a 3 month lead in period prior to transplantation. Patients were re-assessed once per month for 4 months after the initial injection and a final time 2 months after the fourth follow-up visit, for a total follow up time of 6 months. All patients were diagnosed non-familial ALS (sALS). In total, 31 received the MSC injections and 25 were given only riluzole. The incidence of adverse effects between the groups was not significantly different, and all effects were considered mild to moderate, with the most common effects being influenza like symptoms, back pain, and general musculoskeletal pain. Four adverse drug events occurred (2 headache, 1 fever, 1 general pain) within 2 days of the first transplantation and were managed and resolved in another 2 days. The mean change in ALSFRS-R during the 4 month follow up in the MSC group was -1.67 compared to -4.67 in the control (p<.001), and remained significant at the 6 month time point (p=.003). This was accompanied by a decreased rate of monthly decline in the 4 month follow up compared to the controls (p=.003) and also at 6 months (p=.018). AALS scores changed by significantly less in the MSC group compared to the control after 4 months (p=.009), however FVC showed no significant difference. Cytokine analysis was performed on 28 of the MSC group participants and showed significantly increased levels of anti inflammatories such as TGF-b, IL-10, and IL-6 and decreased levels of TNF-a and MCP-1. Here multiple injections of autologous MSCs are shown to be effective at ameliorating the effects of ALS and preserving motor function with limited side effects. Critically, FVC showed no significant difference between controls and treated groups. Although AALS contains a respiratory function component, it is possible improvements in other areas of motor function are masking a non-improving respiratory score.48 Post-hoc analysis of survival showed no significant difference between the MSC group and the control. The authors note that this may be due to the very limited presence of MSCs present after 6 months (Figure 2).
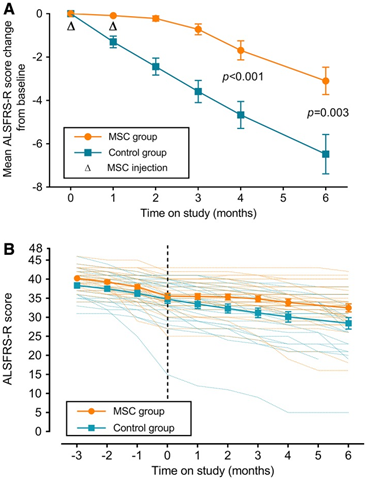
Figure 2 ALSFRS-R scores among MSC treated and control groups. (A) Change from mean ALSFRS-R score (B) Mean ALSFRS-R score for each group over the course of the whole study.47
Using the conditioned media of stem cells has also been suggested to be therapeutically useful, as many neuroprotective NTFs are often secreted by stem cells. Adipose derived stem cells are known to secrete anti-inflammatory factors, anti-apoptotic factors, and NTFs such as BDNF, NGF.49 In an animal trial, adipose stem cells (ASCs) were derived from human adipose tissue and cultured. 200mL of the ASC-CM was delivered to 16 SOD1 mice intraperitoneally every day for 12 days starting at 35 days old. 9 SOD1 mice were injected with a vehicle and served as the control. After day 47 mice were euthanized and tissue from the gastrocnemius was analyzed for NMJ integrity. NMJ’s of the CM treated group were found to have more co-localized neurofilament and a-bungarotoxin, indicating greater NMJ integrity than the vehicle group (p<.05). Here conditioned media proves to be effective at maintaining the NMJs while symptoms of ALS are mild, such as early in the course of the disease and in mild forms. Whether this greater integrity of NMJs translated into quantitatively improved motor performance was not measured.
Lineage- bone marrow cells are another population of stem cells that can be isolated from adults that consists of progenitors, precursors, and other stem cells.50 In a polish study, autologous samples of Lin- cells from 12 individuals with sALS were injected intrathecally to determine their effects.51 Progression of the disease was monitored primarily by the Norris scale and assessed at baseline and periodically after transplantation. Progression was also monitored by ALSFRS-R score at baseline and throughout the study. NTFs, cytokine levels and miRNA profiles were quantified in patient the blood plasma and the CSF. Two groups were identified in the study, the first group receiving about 12x106 total cells and the second receiving 4.5x106. Group 1 did not show significant neurological decline, measured by Norris and ALSFRS-R scales over the 6 months, whereas the second group did. Analysis of the CSF and blood of subjects from group 1 showed significantly decreased levels of the inflammatory markers C3 and C4 compared to their baseline measurements and also to the subjects of group 2 (p<.05). Group 1 also showed increased amounts of NGF compared to group 2 from the same time points (p<.05). NGF levels were found to reach a maximum 1 week following transplantation where they began to fall and reach a minimum by 6 months post transplantation. Levels of BDNF followed a similar trend, however the differences between the two groups were not significant. In the CSF, both groups showed increased expression of miRNA 206 and 133a (p<.05) and decreased levels of the inflammatory miRNA 378 (p<.05). In the plasma both groups displayed increased amounts of miRNA 155 and decreased amounts of miRNA 378, with group 2 additionally showing increased miRNA 206 and decreases of miRNA 1 (p<.05). Changes in miRNA profile in both the CSF and plasma indicate amelioration of ALS phenotypes (51). Data from this study provides further evidence that autologous bone marrow stem cells can be used to therapeutic benefit. Neuroinflammation was alleviated, evidenced by reduced inflammatory cytokine levels in the CSF, NGF levels were increased, and most importantly motor function decline was stalled for the duration of the study. Another explanation for the differences between the two groups, aside from cell dose, could be the age of the group members. Group 2 had an average age 3 years greater than group 1 and also more marked loss of motor function measured at baseline. These could indicate a decreased effectiveness of autologous samples taken from older subjects and decreased effectiveness in more severe cases of ALS.52
Intramuscular injections of bone marrow MSCs were tested in a group of 40 SOD1 transgenic mice.52 20 seventy-day old mice received 300,000 bone marrow cells isolated from GFP mice injected into the quadriceps femoris. Functional outcomes of treatment were measured by rotarod and treadmill tests. Mice were euthanized 5 weeks-later and their tissues were analyzed for live transplanted cells, ALS biomarkers, and NMJ integrity. Survival rates were calculated by keeping random mice from both the experimental and control groups until disease endpoint, defined as the time when mice were unable to right themselves after being placed on their sides for 30 seconds. While disease onset was unaffected, survival time was significantly increased in the BMC group (p<.005). Progression of motor symptoms was also slowed in the treated group compared to the controls (p<.05). Transplantation also downregulated the expression of certain ALS biomarkers associated with worse survival such as collagen, type XIX, alpha 1, glutathione reductase and sorting nexin 10. Other genes related to metabolic function that are upregulated in ALS were rescued to WT levels. NMJ integrity was analyzed by administration of DiL into the mouse quadriceps 5 days prior to euthanizing. BMC treat mice showed significantly greater NMJ integrity compared to controls (p<.05). In the BMC mice, higher levels of the neurotrophic factors NT4 and BDNF were found (p<.05), indicating that these effects were mediated in some way by these NTFs (Figure 3).
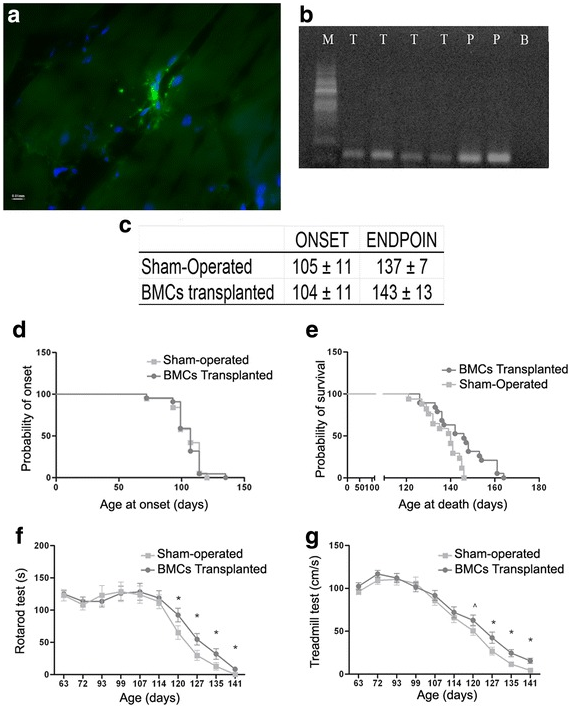
Figure 3 Effect of BMC transplantation into skeletal muscle of ALS transgenic SOD1G93A mice. A) Section of quadriceps femoris stained for GFP cells (green) and nuclei (blue). B) Electrophoresis of quadriceps tissue for the detection of GFP expression after BMC transplantation (T treated mice, P GFP cells used as positive control, B Black control, M RNA Marker). C) Days to disease onset and disease endpoint in both vehicle (sham-injected) and transplantation groups. D) Probability of disease onset as a function of age. E) Probability of survival as a function of age. F) Time to failure in rotarod test as a function of age. G) Maximum speed measured by the treadmill. n = 20 animals in each group. ^p < 0.10; *p < 0.05. Error bars indicate SEM.52
Stem cells are shown to exert therapeutic effects in patients with ALS, extending survival in some cases, decreasing motor neuron degradation, and reducing the decline in motor function attributable to the disease. The two main routes of therapy, replacement and non-replacement, both show promising results in both animal trials and early phase clinical trials. Replacement therapies involving neural stem cells, either harvested from fetuses or differentiated from ESCs/iPSCs have been able to be transplanted, differentiate, migrate, and survive in the diseased ALS microenvironment, where they were able to reduce motor function decline, alleviate neuroinflammation, and release neurotrophic factors, exerting a protective effect. NSCs were observed across all trials presented to be free from tumorigenic activity, and the transplantation procedure producing only mild to moderate adverse effects. While some animal trials linked increased survival times to treatment with NSCs, a phase I clinical trial was unable to make such a connection. Factors influencing this are the dosing in a phase I trial. Cell dosages are not necessarily designed to be of therapeutic concentration, as the primary goal is to assess the safety of their use. Another factor is the follow up period of the trial. Longer term follow ups will allow for greater accuracy in the determination of the effects of treatment on survival. Replacement therapies involving NSCs also bring with them certain ethical issues around the use of tissues derived from ESCs, miscarriages, or abortions, however these could be skirted by either differentiating NSCs from induced stem cells, or by opting for a non-replacement strategy. Non-replacement strategies have also been shown to effectively reduce the severity of ALS in both animal models and clinical trials. The main benefit of non-replacement trials is their flexibility. Efficacy ahs been shown in both intrathecal and systemic injection methods, offering greater flexibility of deliver. Non-replacement therapies are also very flexible in the source of stem cells, including dental pulp, adipose tissue, and most commonly bone marrow. Autologous samples of bone marrow have also been tested and shown to be effective, however these effects are possibly reduced by the diseased microenvironment. More research is required to fully determine these effects. Overall, non-replacement therapies are shown to release NTFs, reduce inflammatory cytokine levels, increase anti-inflammatory cytokine levels, preserve neuromuscular junction integrity and motor function, and also be safe to administer intrathecally, intraspinally, intramuscularly, and intravenously with no serious adverse effects. Effects on overall survival require more research to fully determine as some studies report and effect while others do not. Between the two types of treatments the most problematic metric related to survival appears to be forced vital capacity, FVC. This indicates that while motor function decline is retarded in the limbs, the same does not apply to muscles involved in respiration. Ideas for future studies would include investigating the interaction between stem cell therapies and forced vital capacity. Because non-replacement therapies are able to be delivered by means other than intrathecal/intraspinal injection, combination therapies of MSCs and NSCs seem plausible. The NSCs would be able to support and supplant host cells, astrocytes primarily, while MSCs would be able to secrete NTFs to support those cells’ survival and also modulate the immune response of astrocytes to promote a greater return to normal function. Possible future trials would also include combined trials to determine the safety and efficacy of these treatments.
None.
None.
Authors declare that there is no conflict of interest.

©2020 Crisologo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.