Journal of
eISSN: 2475-5540


Research Article Volume 4 Issue 2
1Criticare Hospital and Research Centre, India
2ReeLabs Pvt. Ltd, India
Correspondence: Bhagat Rajput, Criticare Hospital and Research Centre, Main Gulmohar Road, Andheri West, Mumbai?400049, India, Tel +91 9820 8001 87
Received: September 30, 2017 | Published: March 14, 2018
Citation: Rajput B, Kulkarni R, Bopardikar A, et al. Retrospective analysis of role of autologous bone marrow derived mononuclear stem cells in the management of degenerative arthritis of knee. J Stem Cell Res Ther. 2018;4(2):21-27. DOI: 10.15406/jsrt.2018.04.00109
Background: Osteoarthritis (OA) Knee is the most common disease of the joints and a leading cause of chronic disability, especially in the aged population. The pathogenesis of OA knee has been linked to biomechanical and biochemical changes in joint cartilage, this study is to expand the literature on safety and efficacy of BM−MNCs in treatment of OA knee.
Method: This study was carried out to investigate the effects of intra articular injection of autologous BM MNCs in the patients with osteoarthritis of knee. 11 patients were selected from the age group of 45−75, Bone marrow was aspirated from each patient and BM−MNCs were enriched in the laboratory. All suspensions were tested for microbial contamination before injection. The patients were followed up for a period of 1 year with 1 month, 3 months, 6 months and 1 year intervals. The VAS, WOMAC and Walking distance scores were assessed along with x−ray and MRI of affected joints.
Results: A positive response to the BM−MNC injection was observed in more than 70% of cases after 1 month of injection. We found no adverse events during 1 year follow up with the significant decrease in VAS score which decreased from 73.81 to 35.09 average at 1 year, similarly WOMAC score also decreased substantially in the same period from 76.54 to 41.36 average. The walking distance also improved with more flexibility of joints after BM−MNCs administration. The mean walking distance increased to136 meters from baseline of 39.09 at the 1 year follow up. All assessments were statistically significant.
Conclusion: Autologous BM−MNC’s intra articular transplantation could be a promising way to reduce the signs and symptoms of degenerative arthritis knee, it can be concluded that a second injection or multiple sittings may be required in patients with severe OA or with higher grade OA.
Keywords: bone marrow mononuclear cells, intra-articular, osteoarthritis
Osteoarthritis (OA) Knee is the most common disease of the joints and a leading cause of chronic disability, especially in the aged population.1 The pathogenesis of OA knee has been linked to biomechanical and biochemical changes in joint cartilage, e.g. inability to withstand normal mechanical stresses, limited nutrients and oxygen supply, inadequate synthesis of extracellular matrix components, increased synthesis of proteinases and overall apoptosis of chondrocytes.2–5 Synovial inflammation is a response of synovial macrophages to cartilage debris and catabolic mediators entering the synovial cavity which limits the cartilage repair.6
Current nonsurgical treatment options for OA knee focus on short-term relief of symptoms like physical therapy, activity modification, bracing, oral medications, and intra−articular use of steroids. Although these may be effective in providing some relief of pain, all are short-term measures. Intra−articular injection of Hyaluronic acid (HA) is effective in patients with less-severe OA, but pain relief is limited to a few months.7 Since hyaluronic Acid does not slow down the progression of the disease process, the surgical option becomes inevitable.
Autologous biologic therapies are also promising with early data showing that platelet rich plasma (PRP) injection for OA knee may be of benefit for patients with mild to moderate osteoarthritis.8 Two recent trials of HA versus PRP injections for OA knee demonstrated the superiority of PRP.9,10 However, PRP is less effective for patients with more severe OA.8 Regenerative medicine has gained researcher’s and clinician’s interest as an alternative of surgical treatment for OA knee. The use of autologous BM−MNCs in OA knee to decrease symptoms with the slowing down of underlying disease process is quite promising. The composition of these nucleated cells is diverse including Mesenchymal Stem Cells (MSCs), Hematopoietic Stem Cells (HSCs), Monocyte Precursor cells, Macrophages, T cells, B cells, Dendritic Antigen Presenting Cells, Natural Killer Cells and Neutrophils.11−13 The action of these cells, both in isolation and synergistically, once introduced into the arthritic joint may help in improving the pain and functions by replenishing damaged joint structures and slowing down of catabolic immune responses, thus alleviating the symptoms and progression of the disease.13,14
Early clinical studies using both isolated mesenchymal stem cells and bone marrow aspirate concentrate to treat osteoarthritis have been encouraging.16–19 Stem cell treatments could potentially be a safe, less invasive, and nonsurgical treatment for OA knee however, limited evidence for efficacy of this type of treatment exists in the literature. The purpose of this study is to expand the literature on safety and efficacy and of BM−MNCs in the treatment of OA knee in a hospital setting in India.
This study was carried out in two hospitals to investigate the effects of intra articular transplantation of autologous BM−MNCs in osteoarthritis of knee. After initial screening of the patients, the patients were included in the trial that had mild to moderate osteoarthritis of the knee. These patients had pain and stiffness of knee joints while asymptomatic at rest. All patients were investigated to exclude other causes of knee pain. MRI and X−ray of knee were also done for evaluation. Total 16 patients were enrolled in the study after inclusion exclusion criteria selection, out which 10 patients suffered from Bilateral OA and 6 with unilateral OA knee. The study was started in April 2014 and the assessment data considered for analysis in this article is till March 2017, All 16 patients enrolled in the study received BM−MNCs using a specified treatment protocol described. 5 patient’s data was not analyzed because of irregular follow up intervals and dropouts. Total 11 patient data was collected (7 females and 4 males) prospectively and analyzed retrospectively. The patient data shown in this article is of those patients who have completed one year follow up. Dropouts are not included in assessments.
The study was approved by the Ethics Committee of the Hospitals concerned and the support laboratory. All patients were educated about the procedure by the investigating team and an informed consent was obtained. The consent form was approved by the Ethics Committee of the institution. The study was supported by Ree Labs Pvt Ltd, Mumbai.
Bone marrow derived mononuclear cells (BM−MNCs)
The mixed cell population, consisting of T cells, B cells, monocytes, natural killer cells, circulating endothelial cells (CEC), endothelial progenitor cells (EPCs), Mesenchymal stem cells, hematopoietic stem cells, and multipotent adult progenitor cells.
Objectives of study
Inclusion criteria
The study included patients from both sexes between the ages of 40−75 years with established osteoarthritis of the knee. The diagnosis was confirmed by clinical symptoms and MRI or X−ray of the affected knee joint. Patients were required to have a normal liver and renal function and controlled diabetes (if diabetic). All patients signed a written consent before inclusion in the study.
Exclusion criteria
The patients who had any structural defects, or any other cause of leg pain or had type of arthritis other than degenerative were excluded from the study.
BM−MNCs isolation
Prior to the procedure the patients were stopped from taking corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs) for at least 2 weeks as these medications can reduce healing.20‒23 All patients underwent a bone marrow aspiration under local anesthesia, about 40–60ml of bone marrow suspension was harvested from posterior iliac crest of donor and captured in a 50ml tubes (Falcon, BD Bioscience) containing heparin (10 U/ml) to prevent clotting. Collected bone marrow samples were transported to processing laboratory in cold chain and samples were immediately processed upon receiving. BM MNC cell isolation was done using previously stated procedures with some modifications, The aspirates were mixed with hyroxyethyl starch (HES) in the ratio of 1:3 and gently shaken for 10minutes in a mechanical shaker (REMI), After mixing the sample with H.E.S it is kept for sedimentation for 30minutes, the supernatant was collected in 50ml conical tubes (corning) and centrifuge it at 1500RPM for 20minutes. Pool the pellet to make up the volume to 30ml, the volume was loaded on to the 15ml Ficoll Hypaque (Histopaque Sigma), and Centrifuge again at 1500RPM for 20mins. Supernatant was discarded and the buffy coat was collected and washed twice with sterile PBS. The MNC count and viability of the isolated cell population was determined by Hemocytometer using trypan blue dye. The final volume of 3ml was prepared using Plasmalyte A (Baxter) and was sent to the hospital for intra articular injection. In case of both knees the final volume was made upto 6ml (3ml per knee). However the isolated cells were not characterized, as the study was only to check the safety and efficacy of bone marrow isolated mixed population of cells. In next phase the cells characterization can be considered before injection into patients. Sterility testing of each isolate was done before injection.
BM MNCs therapy
Standard intra articular injection methods were used to inject cells into the affected knee joint using ultrasonography guidance. All procedures were done in the radiology suite under strict aseptic conditions and there were no complications during the procedure. All patients were advised to avoid weight bearing in the injected limb for three weeks. Minor joint pain was expected and patients were advised that cold compression may be used to control the pain. However no patient had any specific complaint in this regard.
Follow up
All patients were followed up at 1 month, 3 months, 6 months and 1 year. During each follow up visit the patients were questioned regarding adverse events, Walking Distance measure, VAS and WOMAC scoring. All patients had a routine blood count and biochemistry at each visit. However Imaging was only done at the last follow up visit at 1 year and included AP and lateral X rays of the knee and a MRI study of the affected knee joint.
Statistical analysis
All data are expressed as means and standard deviations. SPSS software was used to analyze statistical differences between preoperative and follow-up values of WOMAC scores, VAS pain scores, and Walking Distance scale. For all tests, P value significance was defined as P<0.001.
Results
Our final sample size for analysis was 11 patients (7 female and 4 male); the mean age was 61.2years (Age range 45 to 75 years 63.63% female and 36.36% male) (Figure 1). Bone marrow was aspirated from posterior iliac crest of each patient and mononuclear cell population was enriched using gradient separation method using Ficoll hypaque (Table 1). After enrichment the cells were sent to hospitals and Intra articular injections were administered to respective patients. No complications or adverse events were observed during the administration process or during 1 year follow up.
Patient |
Age of the patient |
Gender |
Bone marrow volume |
Cell count (millions) |
1 |
64 |
Female |
45ml |
298 |
2 |
58 |
Female |
50ml |
310 |
3 |
45 |
Male |
40ml |
275 |
4 |
64 |
Female |
75ml |
390 |
5 |
68 |
Male |
55ml |
300 |
6 |
75 |
Female |
47ml |
286 |
7 |
58 |
Male |
40ml |
250 |
8 |
55 |
Male |
60ml |
356 |
9 |
61 |
Female |
50ml |
280 |
10 |
64 |
Female |
45ml |
261 |
11 |
62 |
Female |
52ml |
299 |
Table 1 Demographic and lab data viz. number of patients, age, bone marrow volume and respective cell counts
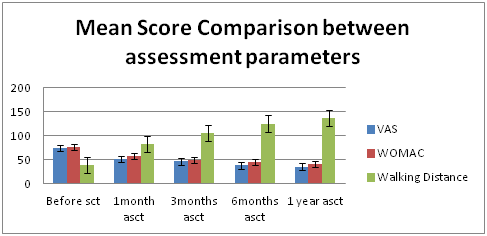
Figure 1 Graph showing significant decrease in VAS and WOMAC Scores and Increase in Walking Distance at different follow up intervals.
A positive response to the BM−MNC injection was observed in more than 70% of cases after 1 month of injection. The follow-up period was 1 month, 3 month, 6 months and 1 year (Table 2) (Figure 2). Visual Analog Score (VAS) for pain decreased promisingly throughout the follow-up period, the mean score, before BM−MNCs administration, 73.81 points decreased to 35.09 points over a period of 1 year, was statistically significant (P < 0.001). Similarly WOMAC score decreased substantially over the study period which was 76.54 points before BM−MNCs administration, but decreased to 41.36 points at the 1−year evaluation. The differences between the pre BM−MNCs administration WOMAC score and those obtained at the 1 year follow−up points after the after BM−MNCs administration were statistically significant (P< .001). The walking distance in meters assessment also showed positive outcome with patients showing increased walking ability after BM−MNCs administration. The mean walking distance before BM−MSC was 39.09 meters which improved to 136meters at the 1 year follow up with p value < 0.001 (Table 3) (Figure 3).
|
Pre SCT |
1 Month |
3 Months |
6 Months |
1 Year |
VAS (mm) |
73.81 ±7.73 |
51.54±9.08 |
46.36±9.15 |
38.09±8.52 |
35.09±7.67 |
WOMAC |
76.54±7.6 |
57.27±9.58 |
49.81±8.29 |
45.27±7.02 |
41.36±7.00 |
Walking Distance (m) |
39.09±7.27 |
82.54±12.96 |
105.27±10.64 |
124.72±15.12 |
136±22.72 |
Table 2 Mean and SD score chart for each Assessment scale
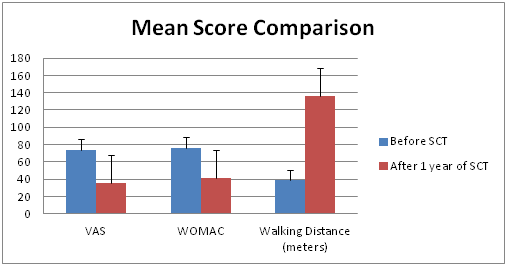
Figure 2 Graph showing significant decrease in VAS and WOMAC Scores and Increase in Walking Distance between before stem cell injection and after 1 year follow up.
Assessment method |
Before SCI |
After 1 year of SCI |
P-Value |
|---|---|---|---|
(Mean ± SD) |
(Mean ± SD) |
||
VAS SCORE |
73.81 ±7.73 |
35.09±7.67 |
<0.001 |
WOMAC |
76.54±7.6 |
41.36±7.00 |
<0.001 |
Walking Distance |
39.09±7.27 |
136±22.72 |
<0.001 |
Table 3 Clinical outcome Assessment of Pre Stem cell injection and follow up of 1 year with P−Values
VAS, Visual Analogue Scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; SCI, Stem cell Injection
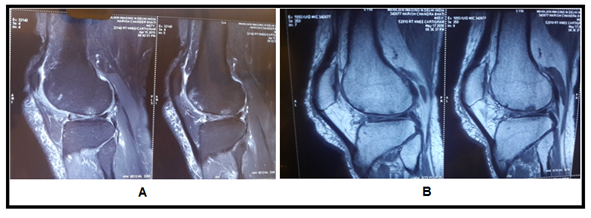
Figure 4 (A) MRI Images show subchondral cyst in femur with multiple breach in articular cartilage, (B) images show disappeared subchondral cyst with healed cartilage breach at one year follow up after stem cell transplantation.
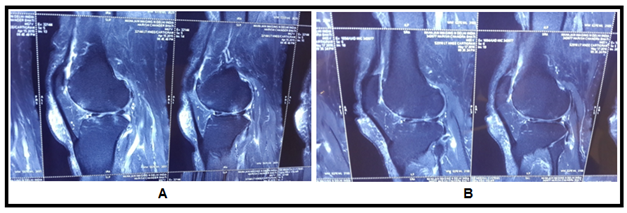
Figure 5 MRI Images showing healing pattern in cartilage breach. (A) MRI Images showing more breach in articular cartilage before stem cell transplantation, (B) images show reduced breach in articular cartilage after stem cell transplantation.
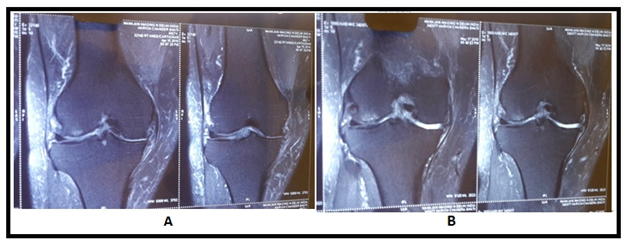
Figure 6 (A) MRI image shows reduced joint space with degenerated cartilage, (B) image shows increase in cartilage thickness.
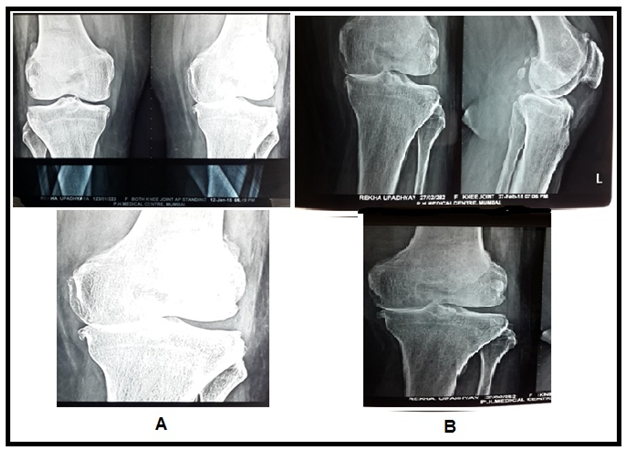
Figure 7 X ray Images showing cartilage regeneration. (A) Image showing lost cartilage in medial joint space in left knee (B) image showing regenerated cartilage in the same joint.
The MRI study at the last follow−up visit (1 year) showed an important improvement in the cartilaginous tissue in different parameters. Patients showed partial restoration of medial joint space of knee with disappearance of subchondral bone edema with increased thickness of articular cartilage which was more smooth and non porous in contrast to pre BM−MSC transplant stage. The pre and post BM−MNCs transplant MRI/x−Ray studies showed notable changes in cartilage defects (Figures 4‒8).
Although the potential for BM−MNCs to regenerate articular cartilage has long been recognized, very few clinical trials have been performed. In most clinical trials, the mode of introduction of MNCs into affected joints was by surgery and therefore an invasive procedure. The other route of MNC’s introduction could be intra-articular injection of the cells. In this context, Centeno et al.24 performed a clinical trial on one patient and reported the intra−articular injection of expanded MSCs as a safe procedure without any complications.24 Another report emphasized safety of this method belonged to Davatchi et al.25 who examined the expanded MSC’s potential on four OA patients with six months follow-up after cell injection.25 In the present investigation, we have reported the results of 1 year follow up of intra articular injection of BM−MNCs with the main purpose to show that it is a safe and feasible procedure. As a secondary objective the patients were followed up to assess the efficacy of this procedure. We mainly relied on clinical parameters which included the VAS and WOMAC scores and Walking Distance assessments. Although the X-rays and MRIs were also assessed but quantifiable data could not be collected for these investigations. All patients showed an improvement in their pain scores with improvement in the daily activities of life as measured by the WOMAC scoring. At 1 year follow up there was a significant improvement in these scores which suggests that the BM−MNCs are successful in improving the quality of life though quantifiable evidence of cartilage growth and regeneration could not be obtained. Regarding patient’s ability to walk, there was significant improvement till 1 year post injection but some patients complained of decrease in distance by the fag end of 1 year, these findings may be suggestive of requirement of second dose of MNCs at one year. Davatchi et al.25 have reported a high improvement in subjective parameters.25 According to their findings, physical parameters showed much less improvement (i.e., X−ray images). In the present study, we examined the patient’s knee with MRI & X ray images which were not performed in the previously mentioned study. According to images, cartilage thickness appeared to be increased with increase in medial joint spaces in many patients, which indicated definitive cartilage regeneration.
BM−MNCs participate in repair of damaged cartilage in OA knee. To find out the exact result of the repaired or regenerated tissue, histological examination/biopsy was necessary. This was not performed in the present study due to the ethical issues associated with human studies.
However in this study rigorous evaluation of the objective parameters for more than 1 year or multiple doses were not done and thus a definite opinion is not possible. The reason for publishing this study was to show that intra−articular injection of BM−MNCs is feasible for knee osteoarthritis and can lead to substantial improvement in the clinical parameters in OA patients. However this is a very small scale study and has not done quantification of the objective evidence. It is evident that good evidence of the efficacy of BM−MNCs can only come from a larger clinical trial which needs to be organized. Other shortcomings of this study are the nonrandomized character of the study and lack of objective evidence of cartilage growth which is the basic hypothesis of the mode of action of the cells used in this study.
In conclusion the study has laid the foundations for a more detailed study which may be able to clarify these issues. BM−MNCs in OA knee would be a promising way to reduce the signs and symptoms of this disorder and lead to patient satisfaction. Furthermore, this procedure is appropriate for large scale studies aimed to evaluate the regeneration potential of BM−MSCs in the cases of osteoarthritis knee. According to our results, all evaluated parameters appeared to progressively improve till 1 year follow up post−injection. For this reason, it can be concluded that a second injection or multiple sittings may be required in patients with severe OA or with higher grade OA.
None
Author declares there is no conflict of interest.

©2018 Rajput, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.