Journal of
eISSN: 2475-5540


Review Article Volume 2 Issue 6
Department of Zoology, DDU Gorakhpur University, India
Correspondence: Ravi Kant Upadhyay, Department of Zoology, DDU Gorakhpur University, Gorakhpur, 273009, UP, India
Received: April 24, 2017 | Published: May 30, 2017
Citation: Upadhyay RK. Role of calcium bio-minerals in regenerative medicine and tissue engineering. J Stem Cell Res Ther. 2017;2(6):166-175. DOI: 10.15406/jsrt.2017.02.00081
Present review article emphasize role of biominerals in regenerative medicine and tissue engineering. Among all biominerals calcium is essential for body growth and development. It also performs many fundamental functions in cellular metabolism. Inside cell organic matrix is calcified by calcium phosphate minerals. It also embeds bone cells which participate in the maintenance and organization of bone. This article also emphasizes use of hydroxyapatite a natural mineral used as a bone-building supplement with superior absorption in comparison to calcium. It also explains use of scaffolds that mimic the structure and composition of bone tissue and cells. It also signifies use of HAc microparticles or microparticles loaded with PL, superparamagnetic iron oxide nanoparticles, composite scaffolds of nano-hydroxyapatite (nHAp) and silk fibroin (SF) in bone regeneration mainly in osteoregenerative therapy. For better and successful bone regeneration there is a need to develop low cost sintered hydroxyfluorapatite discs to support cellular proliferation and colonization, tailored mineralization, cell and drug delivery. All adhesion components should show low immunoreactivity and high biocompatibility with natural bone tissues. There is an essential need to make new biocompatible materials for scaffolding, biominerals and cementing formulations for regeneration of bones, cranio-maxillofacial, dental and orthopedic surgery.
Keywords: regenerative medicine, silk fibroin, osteoregenerative therapy, ecm
Human body needs minerals as micronutrients because these are required to perform various biochemical functions which are necessary for life. Body organs are composed of various tissues and cells and possess micro elements in ECM which perform structural functions and provide stiffness to the cytoskeleton, bones, muscles and cartilages. Plants receive these minerals from soil and all organisms including man rely on plants. Another source of mineral nutrients is drinking water. Most required minerals for human body are calcium, phosphorous, potassium, sodium and magnesium. Trace elements are also required by micrograms for metabolism. Among trace elements iron, zinc, cobalt, copper, manganese, molybdenum, iodine and selenium are specifically required for normal biochemical functions of the human body. Among all five major minerals calcium is essentially required by children, sub-adults and adult male and females. Calcium makes nearly 99% minerals in bones and teeth, and the other 1% in extracellular fluids, intracellular structures and cell membranes.1 Second element which is essentially required is phosphorus that makes up about 1% of a person's body weight.2 Potassium, sodium, chlorine and magnesium make up only about 0.85% of the weight of the body. Together eleven chemical elements make up 99.85% of the body and required as micronutrients.3,4 Other minerals include non-organic salts of magnesium, potassium, fluoride and citrate in the trace in trace elements4 (Table 1).
Bio-mineral |
Form |
Origin |
Biomedical Use |
Safety Standard |
Calcium phosphate (CaP) |
Calcium Phosphate hydroxylapatite,dahllite, octa-calcium phosphate |
Endoskeleton, ion store, pathological mineralizations and those that are used for the repair of mineralized tissues. Scaffolds, triggering cell differentiation, bone formation, |
Calcium phosphate biomaterials are successfully used in cranio-maxillofacial, dental, and orthopedic surgery. |
High |
CaC2O4.2H2O (weddellite) or as CaC2O4.2H2O (whewellite). |
Tetragonal crystallites, whewellite are acute points and general star-like shape |
Cactaceae species |
and a definite calcium oxalate biomineral seems to be a useful tool for plant identification and chemotaxonomy |
Low |
Autunite |
UO22+ minerals such as (Ca(UO2)2(PO4)2•10-12H2O) or polycrystalline HUO2PO4 |
Autunite is a radioactive orthorhombic mineral which results from the hydrothermal alteration of uranium mineral |
may form thus reducing the mobility of UO22+. Compared to the direct addition of inorganic phosphate to contaminated groundwater |
Low |
Monohydrocalcite calcium carbonate minerals |
CaCO·HO |
Echinoderms and coelenterates, granules in the skin of the holothurian, Molpadia |
biogenic minerals, both in terms of the quantities produced |
Medium |
Amorphous hydrous iron phosphate |
3Fe2O3•2P2O5·10H2O |
Dermal granules of Molpadia intermedia |
facilitates rapid ion diffusion |
Medium |
Hydroxyapatite a natural mineral |
Ca10(PO4)6(OH)2 |
Osteoid matrix |
crystalline complex of calcium and phosphate |
High |
Superparamagnetic iron oxide nanoparticles |
SPIONs |
(SPIONs) are also used in bone regeneration |
superparamagnetic iron oxide nanoparticles (SPIONs), used in drug delivery application, |
Biocompatible High |
Calcium oxides, hydroxides, and sulfides |
CaO and is a white crystalline substance. Ca(OH)2 |
Because calcium oxide does not occur naturally, its production is generally from calcium carbonate or limestone. |
quicklime is in the basic oxygen steelmaking |
Hazardous causes coughing, sneezing, labored breathing. |
Calcium silicate (CS) - |
Ca2SiO4 |
Calcium silicate, also known as slag, is produced when molten iron is made from iron ore |
based materials play an important role in the development of endodontic materials that induce bone/cementum tissue regeneration and inhibit bacterial viability |
sealant to cured concrete or the shells of fresh eggs |
Calcium carbonate |
Calcite or aragonite |
Foraminifera |
exoskeleton,optical mechanical strength, protection, gravity receptors, Ca store |
Low |
Apatite (phosphate carbonate |
Vertebrate teeth (enamel) bone, |
Low |
||
Mg-Calcite |
(MgxCa1x)CO3 |
Foraminiferal calcite |
calcification processe |
Low |
Monohydrate calcite |
CaCO3.H2O |
Calcareous tufa, travertine, stalactite and stalagmite. |
Paints, Inks, Powder Coating and Ceramic Industry |
Low |
Protodolomite |
CaMg (CO3)2 |
Metastable single-phase rhombohedral carbonates |
||
Octacalcium phosphate |
Ca8H2(PO4)6 |
CP-based materials could be good candidates for an advanced material compatible to autologous bone |
Implanted in various bone defects |
|
Brushite |
CaHPO4.H2O |
Occasionally found in dental calculus and renal calculi. |
Brushite kidney stones |
Harmful |
Francolite |
Ca10(PO4)6F2 |
Earthy, pulverulent, foliated |
||
Carbonated-hydroxyapatite |
Ca5(PO4.CO3)3(OH) |
Synthesized starting from calcium nitrate tetrahydrate, diammonium hydrogen phosphate and sodium hydrogen carbonate |
Good biocompatibility and osteointegration of the CHA implant, osteoconductive properties and earlier bioresorption |
|
Graphene-based nanomaterials |
nHA/GLY-CHI composites |
Nanocomplexes for innovative therapeutic strategies and biodiagnostics. |
osteoinductive for human bone marrow mesenchymal stem cells |
High |
Table 1 Showing major role of important bio-minerals in regeneration and tissue engineering
Ocean is a largest reservoir of bio-minerals and all its organisms possess mineralized products as composite materials that are comprised of both mineral and organic components. Living organisms get these minerals through the process of biomineralization. Biomeneralization is mainly formation of calcium-containing phosphate, carbonate, oxalate and other mineral types. This process is also related with hardening or stiffness of the mineralized materials (Table 1). It occurs in many phases as different minerals posses different properties such as shape, size, crystallinity, isotopic and trace element compositions quite unlike its inorganically formed counterpart (Figure 1). Among all biominerals calcium-bearing minerals comprise about 50%5 because calcium fulfills many fundamental functions in cellular metabolism.6–8 This dominance of calcium-bearing minerals makes widespread common usage as calcification. Granules of amorphous hydrous iron phosphate deposited as granules in the skin of the holothurian, Molpadia are alternate of calcium minerals that is available in nature.9 The calcium carbonate minerals are the most abundant biogenic minerals, both in terms of the quantities produced and their widespread distribution among many different taxa.5 Of the eight known polymorphs of calcium carbonate, seven are crystalline and one is amorphous. Three of the polymorphs-calcite, aragonite and vaterite-are pure calcium carbonate, while two-monohydrocalcite and the stable forms of amorphous calcium carbonate-contain one water molecule per calcium carbonate.10
Calcium is major structural element in vertebrate skeleton (bones and teeth) in the form of calcium phosphate (Ca10(PO4)6(OH)2 known as hydroxyapatite (Figure 2). It is key component in the maintenance of the cell structure. It also has important role in bone remodeling and tooth re-mineralization is well known. However, calcium also plays a very imperative role in many biochemical reactions, which are essential for normal functioning of cells. Membrane rigidity, permeability and viscosity are partly dependent on local calcium concentration. The most abundantly produced phosphate mineral is carbonated hydroxyapatite, also called dahllite.5 It is mainly found in vertebrate bones and teeth, as well as in the shells of inarticulate brachiopods. Biogenic carbonate apatite crystals are usually plate-shaped and are exceedingly small i.e. 2-4nm thick and some tens of nanometers long and wide11 (Table 1). Over 99 percent of total body calcium is found as calcium hydroxyapatite ((Ca10(PO4)6(OH)2 in bone and teeth, where it provides hard tissue with its strength. At any time most of the calcium in the body exists as the mineral hydroxyapatite. In plasma 45% of calcium is found in ionized form or physiologically active form, 45% bound to proteins that occur predominantly in albumin. 10% is complexed with anions such as citrate, sulfate and phosphate. It is the most suitable ceramic material for hard tissue replacement implant from the point of view of biocompatibility. It has the chemical similarity with the mineral portions of hard tissue (e.g. calcium+phosphorous).
Hydroxylapatite, also called hydroxyapatite (HA), is a naturally occurring mineral form of calcium apatite with the formula Ca5(PO4)3(OH), but is usually written Ca10(PO4)6(OH)2 to denote that the crystal unit cell comprises two entities. Hydroxylapatite is the hydroxyl endmember of the complex apatite group. Here, the OH− ions can be replaced by fluoride, chloride or carbonate, producing flourapatite or chlorapatite. It crystallizes in the hexagonal crystal system. Calcium and phosphorus are used to form apatite layer. Pure hydroxylapatite powder is white. Naturally occurring apatites can, however, also have brown, yellow, or green colorations, comparable to the discolorations of dental fluorosis. The iron bio-minerals are not included in mineral class because they have significant occurrences as oxides, hydroxides, and sulfides.12,13 Some iron sulfate and phosphate minerals are also reported.14 A second characteristic of biominerals is that many are actually composites or agglomerations of crystals separated by organic material (Table 1). Microcrystalline hydroxyapatite (MCHC) is an excellent form of calcium for building bone. MSHC is mostly calcium and phosphorous. This unique extract contains the bone crystal calcium hydroxyapatite (Figure 5). MSHC also contains about 40 different minerals, amino acids and growth factors in similar proportions to those found in bones.
As bio-minerals are essential constituents of ECS, and skeleton, these are also required for repairing and wound healing of body organs. Hovered, remineralization of grafted tissues, bones and cartilages is highly important for pre and post tissue repairing. It assists the grafts to attain biocompatibility and cellular adhesion. Remineralization of dentin during dental caries is of great clinical interest in which few proteins have their direct role. Dentin matrix protein 1 (DMP1) is a non-collagenous calcium-binding protein that plays a critical role in biomineralization.15 Similarly, amelogenin, the major protein of forming dental enamel, plays a crucial role in the biomineralization.16 It supports intrinsic structural flexibility to accommodate interactions in enamel biomineralization. Transforming growth factor beta receptor II interacting protein 1 (TRIP-1) is an intracellular protein that participates in matrix mineralization.17 It is localized in the ECM of bone.
Bone is a dynamic, hard dense connective tissue. It contains a honey comb like matrix internally, which helps to form bone rigidity and acts as a mineral reservoir. Bone is a mineralized tissue composed of a natural organic matrix strengthened by deposits of calcium phosphate crystals. It is structurally composed of extracelleular matrix and cells. The ECM includes minerals, organic materials and water. The mineral part of the bone matrix is mainly composed of calcium and phosphate in the form of hydroxypatite crystals. Human bone is a modified form of hydroxylapatite18 that is a natural mineral structure which forms crystal lattice of bones and teeth. It is commonly used as a filler to replace amputated bone or as a coating to promote bone in growth into prosthetic implants.19 More exceptionally, modified hydroxylapatite is used for the preparation of artificial bone substances for implants and a large variety of drugs for curing different lesions of bone, soft and mucous tissues of the individual. Microcrystalline hydroxylapatite (MH) is marketed as a bone-building supplement with superior absorption in comparison to calcium.20 It is a second-generation calcium supplement derived from bovine bone.20 Dentin is a calcified tissue of the body and, along with enamel, cementum and pulp is one of the four major components of teeth. Dentin is a bone-like matrix that is porous and yellow-hued material. It is made up of 70% inorganic materials mainly carbonated calcium-deficient hydroxylapatite mineral and some non-crystalline amorphous calcium phosphate. Calcified bone contains about 25% organic matrix (2-5% of which are cells), 5% water and 70% inorganic mineral (hydroxyapatite) (Figure 3). It contains 20-25% organic materials; 90% of which is collagen type 1 and the remaining 10% ground substance that includes dentine-specific proteins. It also contains 10% water which is absorbed on the surface of the minerals or between the crystals. By weight, 45% of dentin consists of the mineral hydroxylapatite, 33% is organic material, and 22% is water. The hardness and rigidity of bone is due to the presence of mineral salt in the osteoid matrix, which is a crystalline complex of calcium and phosphate (hydroxyapatite) (Table 1). Endocrine systems regulate the level of calcium and phosphate ions in the circulating body fluids. Calcium phosphate is used as biomaterial in repair of bone, regeneration of dentin and remineralization of enamel (Figure 3) (Figure 4).
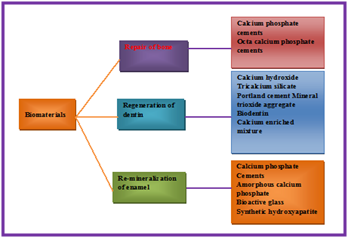
Figure 3 Showing use of calcium phosphate as biomaterial in repair of bone, regeneration of dentin and remineralization of enamel.
Bone tissue is a mineralized tissue of two types, cortical and cancellous bone. Other types of tissue found in bones include bone marrow, endosteum, periosteum nerves, nerves, blood vessels and cartilage (Table 1). Bone tissue contains mineralised matrix made up of an organic component collagen called ossein and an inorganic component of bone mineral made up of various salts. The organic matrix is composed of collagen type I fibers (approximately 95%) and of proteoglycans and numerous non-collagenous proteins (5%). This organic matrix, calcified by calcium phosphate minerals, embeds bone cells, which participate in the maintenance and organization of bone, namely osteoprogenitor cells, osteoblasts, osteocytes, and osteoclasts. Osteoblasts and osteocytes are involved in the formation and mineralization of bone; osteoclasts are involved in the resorption of bone tissue. Modified (flattened) osteoblasts become the lining cells that form a protective layer on the bone surface. Osteoblasts are involved in the creation and mineralization of bone tissue. Osteoblasts are mononucleate bone-forming cells found on the surface of osteon and make a protein mixture known as osteoid, which mineralizes to become bone.21 Osteoid is primarily composed of Type I collagen. Osteoblasts and osteocytes are derived from osteoprogenitor cells, but osteoclasts are derived from the same cells that differentiate to form macrophage and monocytes.21 Within the marrow of the bone hematopoietic stem cells also reside which give rise to other cells, including white blood cells, red blood cells, and platelets.21 Osteoblasts also manufacture hormones, such as prostaglandins, to act on the bone itself.
Bone matrix is slightly monochromate inducting the presence of glycosaminoglycans (CAG) which includes chondriatin sulfate and keratin sulfate. It is mucopolysaccharides with long unbranched polysaccharides consisting of a repeating disaccharide unit. The repeating unit (except for keratan) consists of an amino sugar (N-acetylglucosamine or N-acetylgalactosamine) along with uronic sugar (glucuronic acid or iduronic acid) or galactose. GAGs are responsible for bones comprehensive resistance. These macromolecules play a role in matrix mineralization. Cartilage matrix includes collagen, proeoglycan and non-collgenous proteins. Collagen constituents two third of cartilage dry weight in an adult and it forms interwoven stroma responsible for the tissues tensile strength. Throughout life process of physiological removal, and replacement of bone occurs continuously, without affecting the shape or density of the bone through a sequence of events. All it happens after osteoclasts activation, resorption of bone, osteoblasts activation, and formation of new bone at the site of resorption.22 Bone is constantly renewed by the balanced action of osteoblastic bone formation and osteoclastic bone resorption. These mainly occur at the bone surface. This restructuring process of bone remodeling is important not only for normal bone mass and strength, but also for mineral homeostasis.23
Natural Polymer-Cell bioconstructs are used for bone tissue engineering.24 Bioconstructs substitute the functionality of damaged natural bone structures if critical-sized defects occur. For joining and bound healing of broken bones or for orthopedic and dental applications calcium phosphate bioceramics are widely used. It has a unique characteristic for bone substitution compared with other biomaterials. Porous scaffolds are used for stimulation of bone formation and bone bonding, both related to the specific interactions of their surface with the extracellular fluids and cells, i.e, ionic exchanges, superficial molecular rearrangement and cellular activity. Calcium phosphate scaffolds showed compositional resemblance to bone mineral that they induce a biological response similar to the one generated during bone remodeling. Bone remodeling or renewal is also done for resorption of old bone mineral coupled with the formation of new bone. During resorption the degradation products of calcium phosphate bioceramics such as calcium and phosphate ions are naturally metabolized and they do not induce abnormal calcium or phosphate levels in urine, serum, or organs mainly liver, skin, brain, heart, kidney, lung, and intestine.25 Naturally porous calcium phosphates biomaterials was found more relevant scaffold candidates in bone tissue engineering26,27 (Table 1). Technically, for having better constructs several procedures have been developed to tailor the scaffolds, such as rapid prototyping,28 phase mixing,29 use of porogenic agents,30 or shape replication.31 It depends on selecting and/or combining calcium phosphate phases, resorption kinetics and stimulation of bone formation.32–37 Calcium phosphate biomaterials are successfully used in cranio-maxillofacial, dental, and orthopedic surgery. They are of synthetic origin (obtained after aqueous precipitation or after sintering) or natural origin (freeze-dried or banked bone and derived coral hydroxyapatite), and they are used as bone fillers in the form of cement or granules. As they cannot replace as such the load-bearing functions of bone because of their lower mechanical properties, they are also successfully used as coatings on metallic hip and dental implants in clinics.38
In regenerative medicine and tissue engineering stem cells with multipotent and self-renewal abilities (HPSc and MSCs) also play a vital role to make bio-artificial constructs in culture media. Stem cells grown in specific micro niche assist to make tissue reconstructs through specific cellular differentiation and secretion of various bioactive macromolecules. For cellular proliferation and differentiation of stem cells special support materials are required. Sintered hydroxyfluorapatite discs are used to support cellular proliferation and colonization. Scaffolds that mimic the structure and composition of bone tissue and cells play important role in bone tissue engineering applications. But these should possess proper composition and biocompatibility both in artificial cultures and in vitro tissue grafting. More specifically, only natural polymer-based scaffolds consisting in proteins, polysaccharides, minerals, growth factors etc, are used because they support and enhance interaction between scaffolds and cells. However, to make more functional bone forming smart biomaterials and transplantable composite grafts desirable tissue bio-constructs are to be generated in vitro. Further, to regenerate bone tissue maintenance angiogenic potential of grafted cells is highly required. The other components which are required to generate three-dimensional tissues like fibrin, thrombin, collagen, hydroxyapatite, and beta-tricalcium phosphate in different compositions and concentrations. Phosphate-based glass is also a potential biomaterial that is used for bone repair because of its degradation properties. It control and allow the release of various elements to promote osteogenic tissue growth39 (Table 1). Degradation of zinc containing phosphate-based glass is used as a material for orthopedic tissue engineering mainly to replace lost/degenerated tissues or organs. For wound healing calcium oxide can be replaced with zinc oxide in proportion less than 10 % put a positive effect on bone forming cells.
Osteoprogenitor cells play a significant role in the growth or repair of bones. These are potential cell sources for regenerative medicine and bone tissue engineering but control of their specific differentiation into bone cells is an important issue. BMP-2 grafted nHA/PLGA hybrid nanofiber scaffold stimulates osteoblastic cells growth.40 It is also used as a nanodrug carrier for the controlled and targeted delivery of BMP-2 which enhances bone tissue regeneration and used in treatment of various bone-related diseases.40 Similarly, electrically conductive scaffold act as the skeleton of stem cell niche in regenerative medicine.41 In addition, injectable calcium phosphate/hyaluronic acid microparticle system is also used for platelet delivery for bone regeneration42 (Table 1). Graphene-based nanomaterials are also used as substrates for stem cell (SC) differentiation.43 Osteogenic differentiation of human mesenchymal stem cells is stimulated by reduced graphene oxide level. Osteoclast differentiation from human blood precursors can be enhanced by using biomimetic calcium-phosphate substrates.44 Insulin-transferrin-selenium is also play important role in auricular chondrocyte proliferation and engineered cartilage formation in vitro.45 It helps to retain chondrogenic phenotypes, and promoted engineered cartilage formation when combined with FBS, which is potentially used as key supplementation in auricular chondrocytes and engineered cartilage culture. Despite the biocompatibility and osteoinductive properties of calcium phosphate (CaP) cements their low biodegradability hampers full bone regeneration. Similar to Ca(2+), extracellular Mg(2+) plays an important role in the functions of the skeletal system. But high extracellular magnesium inhibits mineralized matrix deposition and modulates intracellular calcium signaling in human bone marrow-derived mesenchymal stem cells.46 High extracellular Mg(2+) concentration ([Mg(2+)]e) inhibits extracellular matrix mineralization in hBMSCs in vitro. But a high magnesium environment created by the rapid corrosion of Mg alloys may result in the dysfunction of calcium-dependent physiology processes and become disadvantageous to hBMSCs physiology. Ca(2+) signaling also plays an important role in self-renewal and neural differentiation of embryonic stem cells (ESCs).47 In this mechanism intracellular Ca (2+) mobilization is mediated by RyRs (ryanodine receptors); by cADPR (cyclic adenosine 5'-diphosphate ribose) and CD38 (cluster of differentiation 38/cADPR hydrolase); and by NAADP (nicotinic acid adenine dinucleotide phosphate) and TPC2 (two pore channel 2). Similarly, excitation-contraction coupling (ECC) bridge between cardiac electrical activation and mechanical contraction is also driven by the influx of Ca(2+) across the sarcolemma. Because it triggers Ca(2+) release from the sarcoplasmic reticulum (SR) - a process termed Ca(2+) -induced Ca(2+) release (CICR) - followed by re-sequestration of Ca(2+) into the SR. The sodium calcium exchanger (NCX) inextricably couples the cycling of Ca(2+) and Na(+) in cardiac myocytes. However, neuronal sodium channels are important components of the nano-machinery of cardiac calcium cycling.48 Similarly, CaM-dependent protein kinase IIα (CaMKIIα) acts in calcium signaling mechanism in ischemic injury.49 However, to maintain cytosolic calcium concentrations and generate spontaneous calcium oscillations in mesenchymal stem cells electric pulses are used.50 For improving and accelerating the differentiation and functional maturation of human stem cell-derived neurons extracellular calcium and GABA play important role.51 Monolithic calcium phosphate/poly(lactic acid) composite versus calcium phosphate-coated poly(lactic acid) are also used to support osteogenic differentiation of human mesenchymal stromal cells.52
Biocomposite nanomaterials are used in bone bounds and healing. These are on high demand of various biomaterials at global level. Moreover, nHA/GLY-CHI composites are used in tissue engineering of bone, cartilage and periodontal. These composites are osteoinductive for human bone marrow mesenchymal stem cells (HBMS) and have wider application in tissue engineering53 (Table 1). Composite scaffolds of nano-hydroxyapatite and silk fibroin are used to enhance mesenchymal stem cell-based bone regeneration via the interleukin 1 alpha autocrine/paracrine signaling loop.54 These scaffolds promote bone regeneration mainly through cell signaling pathway associated with cell-biomaterial interaction. Though, some other soluble factors also play a role in osteoinduction with nHAp-SF.54 Superparamagnetic iron oxide nanoparticles (SPIONs) are also used in bone regeneration. These assist in stem cell tracking and noninvasive to cells and tissues.55 Similarly, Dex-loaded (dexamethasone-loaded) carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles are used as intracellular nanocarrier, supplying Dex in a regimented manner and promoting superior ectopic de novo bone formation.56 Glycol chitosan/nanohydroxyapatite biocomposites are also used in bone tissue engineering and regenerative medicine.
Naturally occurring porous calcium phosphate is used for making more relevant scaffold candidates in bone tissue engineering26,27 (Table 1). But it is also (Calcium phosphates CaPs) are among the most utilized synthetic biomaterials for bone regeneration, largely owing to their established osteoconductive and osteoinductive properties.57 CaPs is extensively used synthetic bone graft substitutes, are often combined with other materials. CaPs influence angiogenesis and have wider scope in bone regeneration. Similarly, few cement formulations are also prepared by incorporating HAc microparticles or microparticles loaded with PL (10 and 20wt%) (Figure 5). Similarly, by using calcium, phosphorus, water porous ACP nanospheres are formed, while HAP nanosheets or nanorods by using microwave assisted hydrothermal method (Figure 6). Similarly, hydroxyapatite (HAp) and alumina scaffolds influence behavior of MC3T3-E1 cells in culture. These cell culture scaffolds are required for the control of cell attachment, proliferation, and differentiation in vitro.58 For culturing mouse osteoblast-like MC3T3-E1 cells on novel cell culture scaffolds fabricated using ordered nanometer-sized pores (100, 300, 500, and 1000nm) are most commonly used. Both pore size and constituents of films play a role in controlling the morphology and proliferation rate of MC3T3-E1 cells.58 Calcium silicate (CS) -based materials play an important role in the development of endodontic materials that induce bone/cementum tissue regeneration and inhibit bacterial viability59 (Table 1). MesoCS nanoparticles are potentially useful endodontic materials for biocompatible and osteogenic dental pulp tissue regenerative materials. Dentin phosphoprotein (DPP) activates integrin-mediated anchorage-dependent signals in undifferentiated mesenchymal cells.60
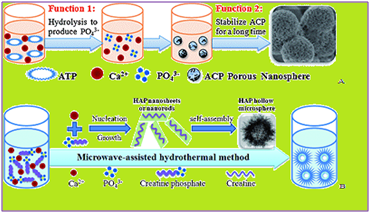
Figure 6 (a) showing formation of porous ACP nanospheres (b) formation of HAP nanosheets or nanorods by using microwave assisted hydrothermal method.
Menin, is a product of the MEN1 gene, that is related to the ontogeny of several cancers such as MEN1 and sporadic endocrine tumors. It is a well known tumor suppressor. Menin interacts with many proteins and involve in various biological functions in several tissues. It plays physiological and pathological roles related to transforming growth factor-beta (TGF-β) signaling pathway in the parathyroid. Menin supports BMP-2- and Runx2-induced differentiation of mesenchymal cells into osteoblasts by interacting with Smad1/5, Runx2, β-catenin and LEF-1, although it has different effects on osteoblasts at later differentiation stages through TGF-β-Smad3 and AP-1 pathways.61 Silk fibroin (SF) is used in osteoregenerative therapy. It combines and gear extraordinary mechanical properties and directs calcium-phosphate formation. The tailoring of the mineralization and mechanical properties of hydrogels through hybridization with FDPs could potentially have a significant impact on cell delivery and bone regenerative medicine.62 The electrically conductive scaffold works as skeleton of stem cell and to maintain niche in regenerative medicine. Biomimesis is used to enhance the quality and function of regenerated tissues. Biomimetic scaffolds increase extracellular matrix, cell-matrix interaction, and tissue-regenerative signaling. These are also used for craniofacial regeneration63 and for replacement of natural body structures of bone tissue as biocompatible grafts. Nacre a micro-architecture is made by using biomimesis that works on a brick-and-mortar arrangement.64 It is an amazing natural nanocomposite that possesses strong mechanical properties and has wider applications in the field of biomedical, biomematerials science and nanotechnology.
Calcium as a biomineral has active role in major cellular functions. Its extracellular level is highly important for various physiological activities such as cellular responses and cell signaling. Calcium homeostasis and ligand-dependent calcium signaling are key components of major cellular responses, including cell proliferation, differentiation or apoptosis.65 Calcium homeostasis is regulation of the concentration of calcium ions in the extracellular fluid [Ca++]ECF. This one of the important mechanism by which the body maintains adequate calcium levels in order to prevent hypercalcemia or hypocalcemia. TRPV1 controls the cellular calcium homeostasis. This parameter is tightly controlled because the calcium ions have a stabilizing effect on voltage-gated ion channels. For instance, when [Ca++]ECF is too low (hypocalcemia), voltage-gated ion channels start opening spontaneously, causing nerve and muscle cells to become hyperactive. The syndrome of involuntary muscle spasms due to low [Ca++]ECF is called hypocalcemic tetany. Conversely, when [Ca++]ECF is too high (hypercalcemia), voltage-gated ion channels don't open as easily, and there is depressed nervous system function. In state of hypercalcemia calcium combines with phosphate ions, forming deposits of calcium phosphate (stones) in blood vessels and in the kidneys. Calcium-sensing receptor regulates cytosolic [Ca (2+)] calcium level and plays a major role in the development of pulmonary hypertension.66 Cytosolic calcium level in primary human gingival fibroblasts is affected by Cyclosporin A and Angiotensin II.67 [Ca++]ECF is influenced by dietary intake, Ca++ absorption in the small intestine, and by excretion of Ca++ in the urine (Figure 7). Importantly, the bones contain 99% of the Ca++ in the body, so bones provide a reservoir of Ca++ that can be used to maintain [Ca++]ECF.
In addition, nutrition factors such as calcium, vitamin D and vitamin K play protective role and assist in integrity of the skeleton.68 It is also influenced by certain trace elements e.g. zinc, copper, manganese, magnesium, iron, selenium, boron and fluoride and negatively by others lead, cadmium, cobalt. Deficiency or excess of these elements influence bone mass and bone quality in adulthood as well as in childhood and adolescence. Micronutrients put beneficial effects on bone homeostasis.68 Vitamin D is essential for calcium and bone homeostasis. Humans are largely dependent on UVB-radiation-induced photosynthesis of vitamin D, as few foods contain vitamin D.69 Parathyroid hormone (PTH) and 1,25(OH)2D (the active form of vitamin D) are important regulators of calcium levels in the body . The major regulator is PTH, which is part of a negative feedback loop to maintain [Ca++]ECF. Parathyroid hormone secretion is stimulated by hypocalcemia, and it works through three mechanisms to increase Ca++ levels (Figure 8). For maintain systemic Ca2+ homeostasis renal Ca2+ reabsorption is essential. It is tightly regulated through the parathyroid hormone (PTH)/PTHrP receptor (PTH1R) signaling pathway.70 In athletes and aerobics exercise-induced decrease in serum ionized calcium (iCa) occurs that trigger an increase in parathyroid hormone (PTH), which stimulate bone resorption.71 Hence, an adequate Ca supplementation before and/or during exercise can fully mitigate the exercise-induced decrease in serum Ca and increases in PTH and bone resorption.71
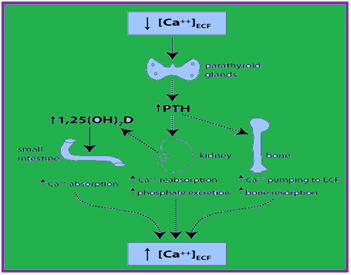
Figure 8 Showing endocrine regulation of [Ca++] ECF (a) PTH stimulates the release of Ca++ from bone, in part by stimulating bone resorption (b) PTH decreases urinary loss of Ca++ by stimulating Ca++ reabsorption (c) PTH indirectly stimulates Ca++ absorption in the small intestine by stimulating synthesis of 1,25(OH)2D in the kidney.
Vitamin D and its' metabolites control whole body calcium homeostasis. These are also part of endocrine system and hormones secreted regulate serum calcium levels but in a very narrow range.72 Production of the vitamin D endocrine hormone, 1,25 dihydroxyvitamin D (1,25(OH)2 D) is regulated by habitual dietary calcium intake and physiologic states like growth, aging, and the menopause.72 In bone healing putative effects are generated by cholesterol, hyperlipidemia, and low vitamin D intake. There are some factors which negatively impact osteoclastogenesis or osteal macrophage activation.73 The calcium associated tissue homeostasis encompasses activities like proliferation, cell death, cell motility, oxygen, and nutrient supply.74 Bone remodeling is stringently regulated by communication between bone component cells such as osteoclasts, osteoblasts and osteocytes. An imbalance of this process is often linked to various bone diseases.75
Excessive bone resorption is responsible for development of bone erosive diseases, including osteoporosis, rheumatoid arthritis, and periodontitis. Natural products are considered best healers for treatment of bone erosive diseases. These affect the mechanism of osteoclastogenesis and bone resorption.76 Bone-resorbing is done by osteoclasts the multinucleated cells, which are differentiated from hemopoietic progenitors of the monocyte/macrophage lineage. The plant origin active ingredients were found efficacious in suppressing osteoclastogenesis and bone resorption. These active compounds are flavonoids, terpenoids (sesquiterpenoids, diterpenoids, triterpenoids), glycosides, lignans, coumarins, alkaloids, polyphenols, limonoids, quinones and others (steroid, oxoxishhone, fatty acid). These natural products exert the inhibitory effects via regulating many factors involved in the process of osteoclast differentiation and bone resorption, including the essential cytokines (RANKL, M-CSF), transcription factors (NFATc1, c-Fos), signaling pathways (NF-κB, MAPKs, Src/PI3K/Akt, the calcium ion signaling), osteoclast-specific genes (TRAP, CTSK, MMP-9, integrin β3, OSCAR, DC-STAMP, Atp6v0d2) and local factors (ROS, LPS, NO). In addition, for better healing of contaminated musculoskeletal defect rapidly-resorbing calcium-sulphate pellets containing amikacin are used to reduce the local bacterial count. These locally-administered antibiotics are used to heal wounds in a limb.77 Further, to reduce inflammation and immunoreactivity modulation of platelet activation and initial cytokine release by alloplastic bone substitute materials is highly important.78
Biominerals are important for cellular and metabolic activities of our body. These are important natural composite material found in mineralized tissue; the bones. Organic matrix, is calcified by calcium phosphate minerals, embeds bone cells, which participate in the maintenance and organization of bone. Calcium phosphates (CaPs) are among the most utilized biomaterials for bone regeneration. CaPs influence angiogenesis and have wider scope in bone regeneration. These show osteoconductive and osteoinductive properties. Molecular control of tissue regeneration for calcium phosphate (CaP)-based materials can be established by defining the parameters critical for tissue induction and those are linked to the molecular circuitry controlling cell physiology. Further, the material properties such as microporosity, ion composition, protein adsorption of a set of synthesized osteoinductive and noninductive CaP ceramics need to be are parameterized and these properties are correlated to a transcriptomics profile of osteogenic cells grown on the materials in vitro. Biomaterial composition and material engineering are important aspects of bone void filling. Advanced space filling methods and strategies are to be explored to make interface between biomaterials and tissue regeneration. Further, new engineering, designs, composites and methods be required for allowing biomaterials to serve as active orchestrators of the molecular and cellular events of tissue regeneration. It will need linking the transcriptional linking landscape of bone induction to biomaterial design parameters.
For biomaterial-induced bone formation certain active ingredients derived from natural plants were found efficacious because they suppress osteoclastogenesis and bone resorption. Calcium homeostasis and ligand-dependent calcium signaling are key components of major cellular responses, including cell proliferation, differentiation or apoptosis. New scaffolds used in tissue engineering, and components of implant devices must biocompatible, transferable and show implantable properties. All biomaterials if mineralized should opt to show high biocompatibility, low immunoreactivity and inflammatory activity. Further, fluoride substitution of HAP will be a better strategy for the development of certain engineered tissue replacements and tissue regeneration systems using ES cells. There is a need to explore more promising biomaterials which can induce necrosis, inflammation and repair, and operate modulation of platelet activation and initial cytokine release and adsorption. For better repair and wound healing composite design, composition, texture and biocompatibility are important features to be considered. For making age specific grafts and tissue implants mathematical modeling should use to reach a parametric fitness and suitability of biomaterial used. Such integrative approaches will prove much advantageous biological process of bone regeneration inside CaP scaffolds.
None.
The author declares no conflict of interest.

©2017 Upadhyay. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.