Journal of
eISSN: 2475-5540


Mini Review Volume 2 Issue 5
Department of Stem Cell, The Academic Center for Education, Culture and Research, Iran
Correspondence: Mohsen Sheykhhasan, Researcher at Department of Stem Cell, The Academic Center for Education, Culture and Research, Qom, Iran
Received: January 31, 2017 | Published: May 24, 2017
Citation: Sheykhhasan M. Mesenchymal stem cells and platelet derived concentrates in regenerative medicine. J Stem Cell Res Ther. 2017;2(5):157-160. DOI: 10.15406/jsrt.2017.02.00079
In the recent years, the unique characteristics of mesenchymal stem cells (MSCs), consisting their angiogenesis and vascularization activity, as well as anti-inflammatory, anti-ulcer and immunosuppressive features plus differentiation capability into multilineage cell, have provided huge favorite among clinicians and researchers that theirs experiments has focused on treatment of different diseases. Additionally, Platelet-derived concentrates, including platelet-rich plasma (PRP) and platelet-rich fibrin (PRF), are remarkably being utilized for lesion healing. In this article, we will be discussed a mini-review on the some of the application of mesenchymal stem cells and platelet derived concentrates in regenerative medicine.
Keywords: mesenchymal stem cells, platelet derived concentrates, platelet-rich plasma, platelet-rich fibrin
IL, interleukin; PDAF, platelet-derived angiogenesis factor; VEGF, vascular endothelial growth factor; EGF, epidermal growth factor; IGF, insulin-like growth factor
Mesenchymal Stem Cells
Mesenchymal stem cells are a significant type of adult stem cells that they can extracted from a variety of tissue (Figure 1), including adipose tissue,1-4 skeletal muscle tissue,5 synovial membranes,6 cervical tissue,7 saphenous veins,8 periodontal ligaments,9 Wharton’s jelly,10 umbilical cord blood,11 umbilical cords,12 amniotic fluid,13 dental pulp,14 placentae,15 lung tissue,16,17 liver tissue,18,19 and dermal tissue.19
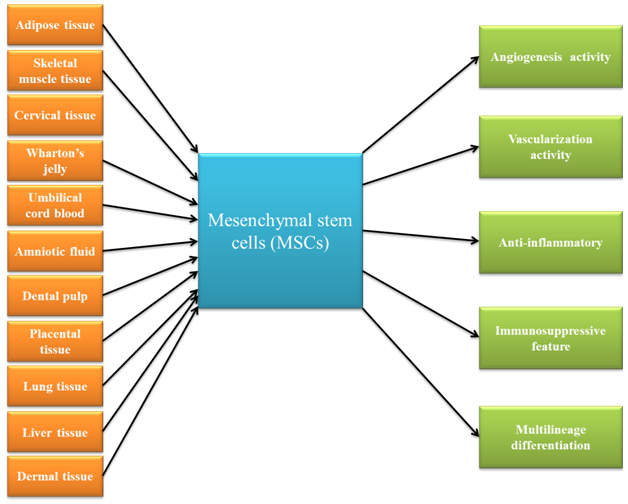
Figure 1 This figure schematically demonstrated that Mesenchymal stem cells can be obtained from a variety of tissue, as well as these cells have unique characteristics.
These candidate cells have some common features, including the secretion of bioactive factors with a protein structure known as paracrine mechanism.20 Paracrine factors secreted from MSCs contain a rich source of valuable bioactive factors, including angiogenesis-related factors, vascularization-specific factors, and neuroprotective factors.21 These factors play an important role in the regeneration process of damaged tissues.21
As a result of its natural properties, MSCs has been suggested for regenerative medicine applications. In addition, these cells have therapeutically valuable properties (Figure 1), such as immunosuppressive and proliferative capacity as well as regenerative potential.22,23
Because of its unique features, MSC is receiving a great deal of attention for disease treatment.22,23
According to information provided by the US National Institutes of Health (http://www.clinicaltrial.gov/), as of Jan 2017, 649 MSC-related clinical trials have either been completed or remain ongoing.24 According to evidence obtained from previous studies, MSC could be used to treat a variety of diseases, including hematological pathologies; Graft-versus-host disease (GvHD);cardiovascular disease;neurological disease;bone and cartilage disease;liver, lung, and kidney injuries;chronic inflammatory and autoimmune diseases; and urological disease.20,24
Due to their potential capacity to induce and enhance hard and soft tissue healing, platelet-derived concentrates are agents with great regenerative capacity, and they arereceiving a lot of attention in the field of medicine.25 The impact of platelet-derived concentrates is the result of their large number of platelets, which includes a wide variety of growth factors and active biomolecules.25
Platelet-rich plasma
PRP (Figure 2), a tiny amount of plasma with a concentration of platelets many times greater than that in the peripheral blood, is obtained by blood sample centrifugation.26 Platelet-derived growth factor (PDGF-AA, PDGF-AB and PDGF-BB), transforming growth factor (TGF-β1 and TGF-β2), platelet factor interleukin (IL), platelet-derived angiogenesis factor (PDAF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), insulin-like growth factor (IGF), and fibronectin are among the key growth factors of PRP that may decrease pain and improve the regeneration process, hence, treating the disease.27
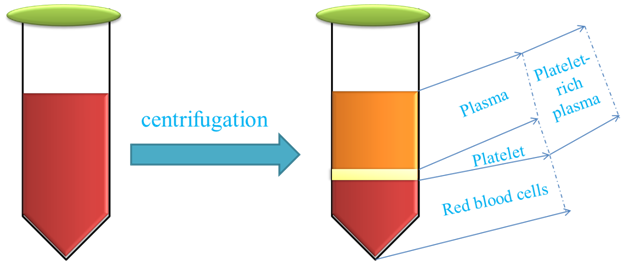
Figure 2 This figure schematically demonstrates the preparation of platelet-rich plasma for regenerative medicine approach.
According to obtained finding so far, it’s identified that PRP could be used to treat a variety of diseases and repair of degenerative or injured tissue, i.e. osteochondral lesions, synovial knee joint failure, acute diaphyseal fractures of the femur, trauma, maxillofacial defects, periodontal tissue augmentation, peripheral nerve injuries, chondral lesions of the hip, wound, degenerative disc disease, femoral neck fractures, oral peri-implant defects, and hair loss.28-42
Previous studies have shown that PRP may have a positive effect on osteochondral lesions.28
It has been reported that PRP applications incorporating biomimetic scaffolds can support soft tissue healing.29 A study performed on synovial knee joint failure demonstrated that PRP has the ability to stimulate bone regeneration as a bone substitute.29
The role of PRP in the healing of acute diaphyseal fractures of the femur was also reported in a study.30
It is clear that platelet biomaterial has an ameliorating effect on a variety of diseases, particularly in the field of skeletal regeneration, such as sinus floor elevation, periodontology, and maxillofacial surgery.31-33. Additionally, periodontal tissue augmentation using mesenchymal stem cells and PRP is currently being performed in the field of regenerative medicine.33 Some of the growth factors-for example, VEGF and PDGF-could be changed in PRP combination after preparation.34 It has been indicated that PRP may increase the regeneration process of peripheral nerve injuries.33 In addition, injectable implants containing PRP may promote tissue regeneration.35 The results obtained from one study illustrate that PRP administration can lead to hernia repair in a rat model.36 PRP has also been used to deliver platelet-related biofactors for disease treatment.37 It is critical for the intended enhancement of many diseases and regenerative potential at the lesion site.37
The combined use of autologous bone marrow MSC and PRF could be utilized for the treatment of chondral lesions of the hip.38 PRP is considered a suitable mixture to regulate the differentiation of bone marrow aspirate into tendon-like cells.38 Additionally, it has been declared that PRP is able to act as a biomimetic approach to the enhancement of wound regeneration. PRP loaded with biogel scaffold can also promote wound healing.37
Moreover, autologous PRP in combination with autologous mesenchymal stem cells may lead to bone regeneration.37 The administration of stromal vascular fraction with PRP plays a therapeutic role in patients with degenerative disc disease.37
Studies have shown that PRP has a clinical effect on endodontic regeneration. Additionally, autologous PRP may play a role in improving femoral neck fractures.39 A pre-clinical experiment demonstrated that the use of PRP in conjunction with MSC can ameliorate oral peri-implant defects.40 PRP is also able to impact the growth-inhibitory activities of pathogenic bacteria.29
Finally, previous findings identified that application of autologous PRP may have a beneficial effect on hair regeneration.42
Platelet-rich fibrin
PRF (Figure 3), a second-generation platelet-derived concentrate, is made by a centrifugation-based method, and there is no chemical material use for PRF preparation.41
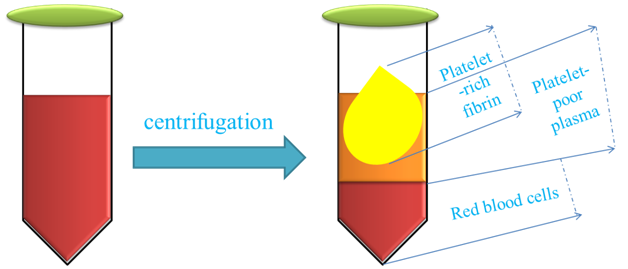
Figure 3 This figure schematically demonstrates the preparation of platelet-rich fibrin for regenerative medicine approach.
Similarly PRP, nowadays, PRF could play an important role in regenerative medicine.43-46 It’s reported that PRF in combination with new bone regenerative flapless technique could be provided an appropriate method for alveolar bone augmentation in patients with horizontal alveolar crests deficiency.43
Recent studies have shown that PRF may have a beneficial effect in improve the dental and periodontal diseases, including implant dentistry, periodontology, oral surgery and regenerative endodontics.37,44,45
It’s indicated that PRF in combination with open flap debridement could provide better periodontal regeneration in terms of conventional flap sites.46 Due to its regenerative potential and angiogenesis activity, PRF may be used to enhance cardiac regeneration.47
It has also been demonstrated that PRF may have a beneficial effect on bone regeneration in a rat model.37 The aim of PRP- or PRF-based therapy is to reduce pain, main or ameliorate tissue function in degenerated tissues, and decrease disability with maximum regeneration of the target tissue.28
The regeneration of degenerative or injured tissue through the administration of MSC has demonstrated hopeful outcomes, which have huge potential as an appropriate therapeutic approach in terms of regenerative medicine. Furthermore, MSCs-related paracrine mechanisms play a critical role in providing the recovery of normal tissue structure and function in defected tissues. Additionally, considering the obtained result in animal and clinical studies, the regenerative potential of MSCs was verified for degenerative tissue restoration. In addition, evidence obtained from a variety of experiments on the effect of platelet-derived concentrates in the field of regenerative medicine is promising and plentiful. Such as, both the PRP and PRF could be used as an excellent option for regenerative medicine approaches. However, in order to identify the beneficial therapeutic properties of the application of MSC and blood-derived concentrates for patients, further clinical trial research should be conducted on the effects of MSC, PRP, and PRF on disease improvement.
None.
The author declares no conflict of interest.

©2017 Sheykhhasan. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.