Journal of
eISSN: 2475-5540


Research Article Volume 2 Issue 5
1Instituto Nacional de Salud P
2Hospital General Regional #1 c/MF, M
Correspondence: Alfonso Carre, Tel (+52) 3293000
Received: January 29, 2017 | Published: May 4, 2017
Citation: Carreón-Rodríguez A, Martínez-Martínez MV, Rodríguez-Valentín R. ET3 promotes the glial phenotype in enteric neural progenitors when used in parallel or after gdnf stimulus. J Stem Cell Res Ther. 2017;2(5):138-143. DOI: 10.15406/jsrt.2017.02.00076
GDNF and ET3 show a complex interaction through the enteric nervous system development. GDNF stimulates the proliferation and differentiation of neural precursors, whereas ET3 blocks differentiation induced by GDNF thereby maintaining a constant pool of precursors. However, ET3 and its receptor play a critical role in the formation of enteric progenitor cells. The effects of these factors seem to compete at times and be complementary in others. Therefore, the aim of this study was to identify the synchronization between these factors to determine the effect on the differentiation and proliferation of neuronal and glial phenotypes in vitro, to generate populations with cell density and state of development suitable for colonization, adaptation and proper growth in transplantation. This work provides evidence regarding the different effects which ET3 on proliferation and neuronal and glial differentiation, depending on at which interact with the action of GDNF.
Keywords: enteric, neural stem cells, neurosphere-like bodies
The gut has its own nervous system division that controls various functions such as motility, secretion, digestion, immune response, sensitivity, etc.1 The cells integrating this system originate from progenitor cells coming from the neural crest and colonizing the entire gut wall during development.2,3
Several studies have established the role of multiple factors on the development of the above mentioned progenitors specially on the following functions 1) the proliferation of neural progenitor cells of the intestine,4 2) the selective induction towards a neuronal or glial fate5 and, 3) the terminal differentiation.6,7 Among these factors, the most relevant of them are Glial Derived Neurotropic Factor (GDNF), Endothelin-3 (ET3) as well as their receptors RET, GFRA1 and ENDRB respectively.
GDNF is a stimulator of the neural progenitors proliferation, but also of their differentiation. Clonogenic cultures of GFP+cells isolated by flow cytometry from dissociation of fetal and postnatal neurosphere-like bodies (NLBs), helped to identify a large fraction of bipotential enteric progenitor cells (EPCs). These cells increased their number under GDNF effect.8
In the presence of a defined medium GDNF also induced neuronal differentiation in both fetal and postnatal culture.8 In this model of clonogenic culture, ET3 blocked both neuronal and glial differentiation reversibly. This effect prevents the commitment of EPCs into either of two ways: neurogenic or gliogenic ensuring the maintenance of a pool of uncommitted, multipotent and self-renewing progenitors. Interestingly ET3 participates in trans-differentiation between pigmented and Schwann cells.9 Barlow and cols., showed in explant cultures as well as in cultures of dissociated cecum and small bowel that ET3 promotes the proliferator effect of GDNF on ENS undifferentiated progenitors. However ET3 inhibits GDNF effect on differentiation.4
EDNRB is the ET3 receptor, and its targeted deletion showed that ET3 was necessary for both the formation of EPCs and NLBs.10 However, some studies have been carried out without ET3 or GDNF as required factors to give rise to NLBs.11 Nonetheless, a gene mutation of ENDRB in lethal spotted rats produces aganglionosis and white coat color in three different species, indicating the importance of this receptor in the formation of intestinal neurons.12
When the progenitor cells from neural crest stop migrating, proliferating or differentiating because of a number of causes such as changes in the expression of GDNF, ET3, or their respective receptors, several types of aganglionosis originate. Among these types of aganglionosis, stand out Hirschsprung disease.13,14
In the normal adult intestine, progenitor cells mentioned above persist as a quiescent pool along life apparently with glial characteristics, which expresses some markers like p75, GFAP, S100B and SOX10.15 This population was initially supposed to be only capable of generating glia in vivo. Now it has been shown to be susceptible to regenerate neuronal loss caused by an insult though this replacement does not occur constitutively.16,17 Nonetheless, it has been shown that intestinal neurogenesis occurs, and this is possibly at least in part to signaling through 5-HT4 receptors.18
The ability for regeneration in vivo could originate from the pool of stem cells, quiescent progenitors, or de novo progenitors. It has gradually opened the possibility to isolate them to generate a population "on demand" with potential for transplantation. Therefore would be possible to replace defects of the enteric nervous system, whether they generated during development (Hirschsprung disease)19 or postnatal life (Chagas, diabetes, poisoning, enterocolitis, etc.).20
Eventually these stem cells or neural progenitors from bowel would represent an option for treatment also for the central nervous system. Transplantation of intestinal progenitor cells is, therefore, a therapeutic option in the medium term. However in order this is feasible several obstacles should be solved first including 1) controlling the in vitro or in situ proliferation for proper cell density and growing rate at the site of transplantation, 2) controlling the fate of differentiation into neuronal or glial subtypes to each region, 3) controlling the establishment of synapses to form glial networks and, 4) controlling the set up of electrical activity and neurotransmitter secretion for full functionality of the system.
Some studies have tried on the isolation and characterization as well as the enrichment of these cells. Metzger and cols., isolated by endoscopy, intestinal epithelial cells from 9 months to 17 years old children. They culture these cells to give rise to neurospheres and then transplanted them into the aganglionic chicken intestine and human hindgut generating ganglionic-type structures, neurons and glia.21
Other studies have exposed enteric progenitor cells to various in vitro environments either organotypic or with factors derived from tissues. Some examples include tissue from the intestine of patients with Hirschsprung disease. These models have shown that neurite outgrowth, neuronal survival, and branching is stimulated in vitro. However failures to do it in vivo suggests the presence of inhibitory factors in the tissue, blocking expression of neural markers (i.e. beta tubulin III, PGP9.5, nNOS, peripherin).22,23
While many of GDNF and ET3 effects on enteric progenitors development have been previously reported as showed above our work was focused in studying the timing of combined effects of these two factors during proliferation and then differentiation in a model able to mimic a kind of in vitro ontogeny to render potential cells with neurogenic and/or gliogenic potential without gene manipulation in order to optimize the success of cell transplantation. Our results show that this model of neurospheres raised with EGF and bFGF and then cultured with GDNF and/or ET3 within a proliferator and a differentiator environment sequentially is suitable for controlling the ulterior fate of bipotential progenitors without gene manipulation.
Animals
Animals used were E14 Sprague-Dawley rat embryos (Harlan) raised at the animal facility of the Massachusetts General Hospital, maintained under standard environmental conditions and fed with rat chow and water ad libitum. Animal care and protocols followed the MGH IACUC guidelines.
Cell culture
Rat fetal intestine at embryonic day 14 was minced and digested for 15min with dispase [250mg/ml]+collagenase [750U/ml]. The tissue was mechanically dispersed and the resulting cell suspension filtered through a 40mm mesh. The cells were divided into one of the four following groups: control (C), GDNF (10ng/ml) (G), Et3 (100nM) (E) or GDNF (10ng/ml)+Et3 (100nM) (GE), seeded at a 10,000cells/cm2 density in low adherence plates (Corning), incubated at 37°C and allowed to grow in Proliferation NeuroCult Medium (StemCell Technologies) supplemented with EGF (20ng/ml), bFGF (10ng/ml) and heparin (0.0002%) for a week. Neurosphere-like bodies (NLB) developed in suspension during this proliferation phase.
At day 7, the number and diameter of NLB's (Feret's diameter) were determined by phase-contrast microscopy and then dispersed with ACCUTASE (StemCell Technologies). Each of the four groups described in the proliferation phase were split in turn into four of the following groups: control (c), GDNF (10ng/ml) (g), Et3 (100nM) (e) or GDNF (10ng/ml)+Et3 (100nM) (ge) seeded at 25,000cells/cm2 density in high adherence plates (Corning) and fibronectin-coated culture dishes (Nunc, LAB-TEK) for immunocytochemistry, incubated at 37°C and allowed to differentiate in Differentiation Neurocult Medium for another week. This scheme of treatment rendered 16 groups classified depending on its treatment during proliferation and then during differentiation phases respectively as follows (proliferation/differentiation): C/c, C/g, C/e, C/ge; G/c, G/g, G/e, G/ge; E/c, E/g, E/e, E/ge and GE/c, GE/g, GE/e, GE/ge.
Histology, immunohistochemistry and immunofluorescence
Cultures were fixed in 4% paraformaldehyde, washed, permeabilized and where indicated stained with hematoxylin and eosin (HE). Primary antibodies used included rabbit anti-Nestin (1:200; Abcam, Cambridge, MA), mouse anti-HuC/D (1:100; Molecular Probes, Carlsbad, CA), goat anti-GFAP (1:500; Dako, Carpinteria, CA, USA), mouse anti-Tuj1 (1:400; Covance, Princeton, NJ, USA). Sections were incubated with primary antibody, followed by biotinylated goat anti-rabbit IgG or goat anti-mouse IgM (Vector Labs, Burlingame, CA, USA) and avidin-biotinylated peroxidase complex (Vectastain Elite ABC kit; Vector Labs). Antibody binding sites were visualized using 4-chloro-1-naphtol (Sigma).
For immunofluorescence, secondary antibodies used included goat anti-mouse IgG Alexa Fluor 594 (1:1000; Invitrogen, Grand Island, NY, USA), goat anti-mouse IgM Alexa Fluor 488 (1:1000; Invitrogen, Grand Island, NY, USA), rabbit anti-goat IgG Alexa Fluor 594 (1:1000; Invitrogen, Grand Island, NY, USA) and rabbit anti-goat IgG Alexa Fluor 488 (1:1000; Invitrogen, Grand Island, NY, USA). Cell nuclei were stained with DAPI (Vector Labs).
Image analysis
An image analysis program (Image J) was used to determine the size of the spheroids according to the Feret’s diameter.
Data presentation
Data were analyzed with Statplus software. Results were calculated as a percentage of control or first-time point value. Data were calculated as the mean±SEM and analyzed by ANOVA, considered significant at p<0.05, followed by Fisher's PLSD test. In general, data correspond to at least three independent experiments.
Spheroids expressed neural markers
Single cell suspensions were prepared from whole intestine to generate NLB cultures under basal proliferating conditions. After seven days in culture, a range of 26-59 spheroids were quantified per plate with a size determined according to Feret’s diameter oscillating from 50 to 250µm. NLBs at this stage were paraffin embedded and then 8µm thick slices were stained with H&E, to evaluate cellularity. Also immunofluorescence against Hu and TuJ1 as neural markers was performed showing that some areas of NLB contained neural bodies although their extensions were distributed throughout the whole volume of NLB. These results demonstrated that under our culture conditions even without a specific factor for neural differentiation, neural cells were already present (Figure 1).
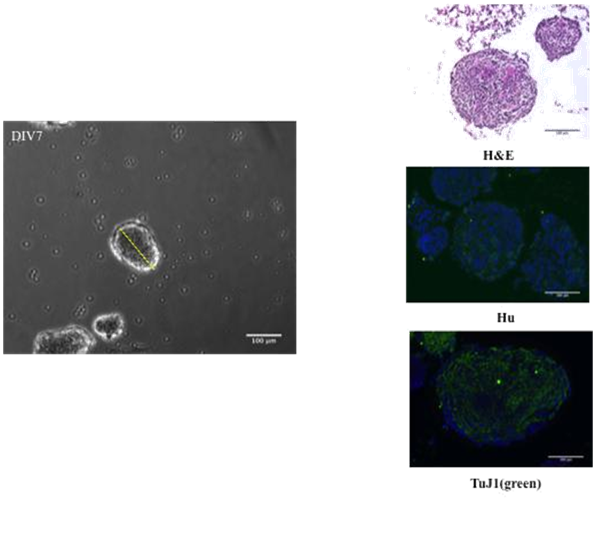
Figure 1 Expression of neural markers on spheroids. Cells from whole intestine of E14 embryo rats, dispersed with dispase/collagenase and plated with EGF, bFGF and heparin. At DIV7 spheroids are ranging 50-250µm in size (Feret’s diameter) and 26-59 spheroids in number per plate.
GDNF increased the number and size of spheroids during proliferation phase
Cell suspensions groups were prepared after dissection to be maintained by seven days in basal proliferating conditions as previously described, under the effect of GDNF (10ng/ml) (G group), Et3 (100 nM) (Et3 group) or their combination (GE group) vs. control group. G group during this proliferation phase gave rise to significantly bigger NLB's according to Feret's diameter when compared to other groups (Control: 163.5±7.1µm, G: 252.9±17.7µm, E: 137.3±7.1µm, GE: 163.2±8.2µm) (p<0.05). There was no significant difference between the numbers of spheroids among four groups (Figure 2).
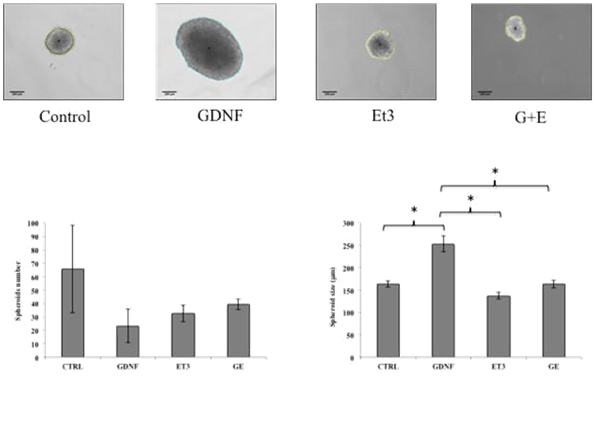
Figure 2 Effects of GDNF and Et3 on number and size of spheroids. No difference in the number of spheroids with any of the treatments, however the average size of GDNF-treated group was significantly higher compared to the other three groups. (Control: 163.5 ± 7.1µm, W: 252.9 ± 17.7µm, E: 137.2 ± 7.1 µm, GE: 163.2 ± 8.2 µm) (p <0.05).
GDNF increased the expression of neural crest origin and phenotype markers during proliferation phase
Spheroids from C, G, E and GE groups were characterized by immunohistochemistry for Nestin or Hu to identify both potentially neural crests derived cells and cells committed to neural phenotype. According to the higher size of spheroids from G group showed above, the number and intensity of positivity for Nestin or Hu were higher too, compared to the other groups (Figure 3).
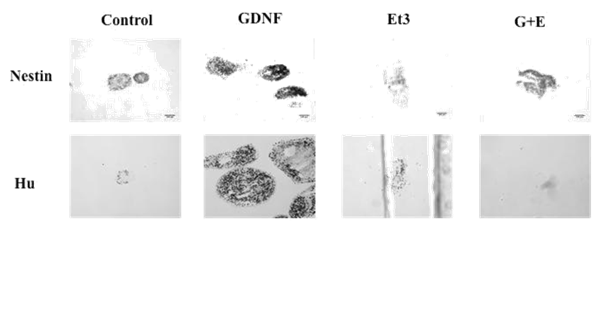
Figure 3 Effects of GDNF on Nestin and Hu expression of spheroids. Nestin (stem cells) and Hu (neural cells) identification by immunocytochemistry shows that expression of these markers is greater in the spheroids treated with GDNF.
GDNF potentiated the number of differentiating cells
After seven days of proliferation phase, spheroids were dispersed and single cells plated at 25,000cells /cm2 density on culture slides (labtek) under differentiating conditions. C, G, E and GE groups were split each one into four groups with the same previous scheme of treatment for 7 additional days, rendering 16 combinations as follows (proliferation/differentiation): C/c, C/g, C/e, C/ge; G/c, G/g, G/e, G/ge; E/c, E/g, E/e, E/ge and GE/c, GE/g, GE/e, GE/ge. At the end of differentiation phase, cells were fixed and stained for Hu or TuJ1 and GFAP. Nuclei were stained with DAPI. C-c group was taken as 100%. Those groups of cells under GDNF effect (in presence or absence of Et3) during proliferation phase increased their number as expected (G/c: 488±66.6 %; GE/c: 416.7±174 %; G/g: 1346.9±373.3 %; GE/g: 1153.1±846.2 %; G/e: 652.6±91.5 %; GE/e: 459.4±222.1 %; G/ge: 1074.5±109.1 % and GE/ge: 1017.3±477.2%). In addition, also during differentiation those schemes of treatment including GDNF produced a significant increase in the number of cells when compared to C-c group (G/g: 1346.9±373.3; GE/g: 1153.1±846.2; G/ge: 1074.5±109.1; GE/ge: 1017.4±477.2%) (p<0.05) (Figure 4A & 4B).
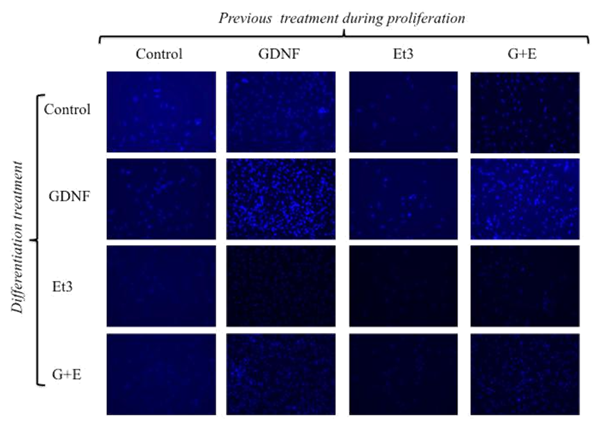
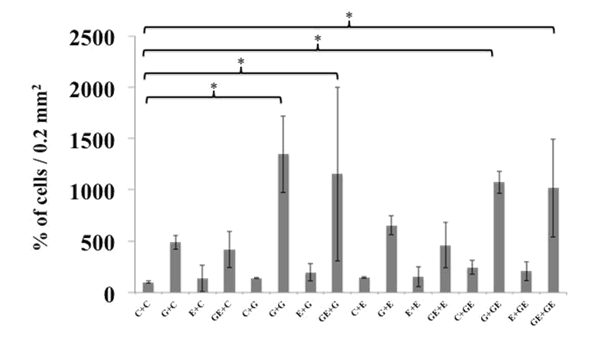
Figure 4 A) Total cell count at the end of proliferation (P)/differentiation (D) process.
Cells were stained with DAPI at the end of the P/D process to determine their amount. The groups G/g, GE/e, G/ge or GE/ge showed higher amount of cells per plate. B) Cell number in the groups G/g, GE/e, G/ge or GE/ge (P/D) was significantly higher when compared against CC (P/D) and the rest of the groups.
When cells were identified by immunocytochemistry regarding their expression of Hu and GFAP was evident that only in those groups exposed during proliferation to GDNF a significant number of Hu positive (neural cells) were present (G/c: 31.3; GE/c: 102.5; G/g: 796.3; GE/g: 1506.3; G/e: 106.3; GE/e: 102.3; G/ge: 601.3 and GE/ge: 1670 respectively). This amount of Hu-positive cells was higher when GDNF was present also during differentiation phase (G/g: 796.3, GE/g: 1506.3, G/ge: 601.3 and GE/ge: 1670). Surprisingly the amount of GFAP positive cells was even higher than those Hu-positive in all cases where neural marker was detected, again when cells were somehow exposed to GDNF (G/c: 55 vs. 31.3, GE/c: 446.3 vs. 102.5, G/g: 1213.8 vs. 796.3, GE/g: 3083.8 vs. 1506.3, G/e: 1002.5 vs. 106.3, GE/e: 782.5 vs. 102.5, G/ge: 881.3 vs. 601.3, GE/ge: 2053.8 vs 1670). Interestingly the average number of GFAP positive cells per field was several times higher than Hu-positive cells in some groups where Et3 was applied (GE/c: 446.3 vs 102.5; GE/g: 3083.8 vs 1506.3; G/e: 1002.5 vs 106.3; E/e: 70 vs 0; GE/e: 782.5 vs 102.5; G/ge: 881.3 vs 601.3; E/ge: 66.3 vs 3; GE/ge: 2053.8 vs 1670) (Figure 5A & 5B).
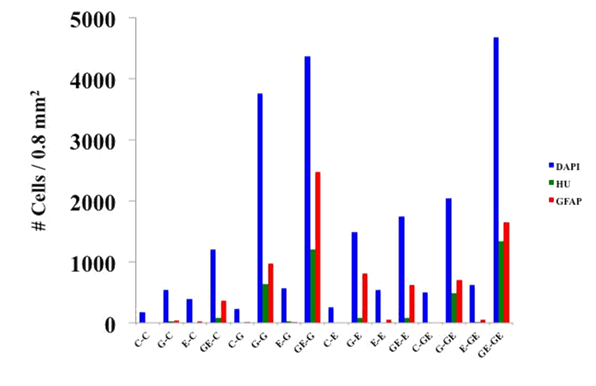
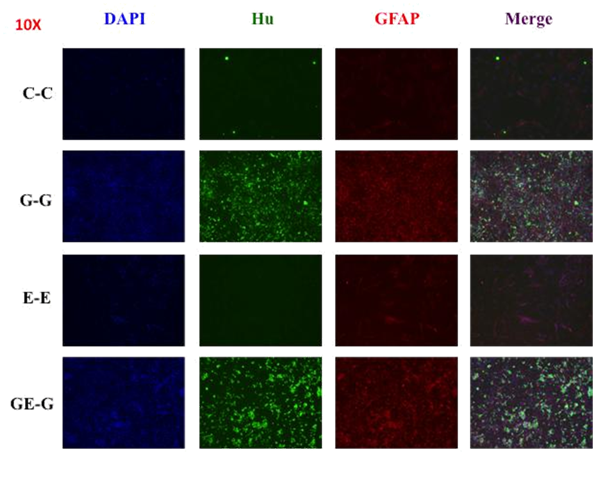
Figure 5 Cellular characterization at the end of proliferation/differentiation process (P/D) (Hu-green/GFAP-red).A) The identification of Hu (neurons) by immunocytochemistryshows that only those treated with GDNF for proliferation induced neuronal differentiation. In each of these groups GFAP expression was consistently higher, especially in the presence of ET-3. B) Some representative images showing the expression of Hu and GFAP predominantly in those groups exposed to GDNF during proliferation and/or differentiation.
In the postnatal gut persistent bipotential progenitors can generate glial cells or neurons depending on certain physiological or pathological requirements (15-17). GDNF plays an important role in proliferation but also during cell differentiation, while ET3 stimulates the proliferative properties of GDNF but blocks its differentiating ability maintaining a constant pool of progenitors. An isolated action of GDNF eventually will produce a whole differentiation of neural progenitors, reducing their proliferation and/or migration, therefore, depleting the pool of stem cells. Moreover, an isolated action of ET3 maintains a pool of stem cells indefinitely without differentiation. The purpose of this study was to explore the interaction between GDNF and ET3 in vitro to optimize for transplantation: 1) cells maintaining the proliferative potential and, at the same time 2) they are susceptible to neural differentiation without depleting the pool of progenitors.
We use a model widely and successfully tested consisting of growing neurospheres with EGF and bFGF as a source of neural stem cells. By histology, we identified the expression of neuronal markers even in the absence of neural stimulation factors according to what was shown by Reynolds & Weiss.24
The serum-free culture under the stimulus of EGF and bFGF was sufficient to generate neurospheres from embryonic gut. The addition of GDNF in these conditions increased the number of cells within the neurospheres, consistent with that reported by Bondurand et al.8 ET3 in our experiments was not a factor required for proliferation and/or survival as Barlow and Bondurand described.4,10 In contrast, the isolated action of ET3 significantly decreased cell proliferation besides reducing the proliferator effect of GDNF.
The proliferation induced by GDNF in the neurospheres is accompanied by an increase in nestin expression as a marker of cell lineage from the neural crest. Also an increase in the expression of neuronal marker Hu was observed, which shows that proliferation is a process that occurs in parallel with the differentiation under the effect of a single neurotrophin without implying that both processes are opposed or alternative. ET3 in this case besides inhibiting proliferation seems selectively block neuronal differentiation as well, and promote by its own glial differentiation as manifested in GFAP expression in cells treated with ET3 even if the expression of neuronal markers is nearly absent. If such an effect on the differentiation toward glial phenotype is due to some other factor present in the SBF that is associated with the action of ET3 to modify its action, it is also something that will need further insight.
Interestingly GDNF also appears to favor the induction of neuronal-glial phenotype according to the proportion of cells expressing GFAP compared to those that express Hu under the effect of this neurotrophin. This effect suggests the actions of GDNF and ET3 affect both proliferation and differentiation of bipotential progenitors mentioned by Bondurand et al.10 but also cannot be ruled out based on our findings that this effect is about two different subpopulations of glial and neuronal progenitors.
The isolated effect of GDNF implied to differentiate all cells at the expense of the pool of progenitors. Therefore, during the differentiation we expected to identify a greater number of neuronal markers without additional modification in cell density. However what we observed was that in addition to increasing positive cells to neuronal markers and surprisingly also glial ones, the effect of GDNF during the differentiation further increased cell density strengthening the notion that proliferation occurs in parallel to differentiation, as the number of markers increases in proportion to the cell density. From these results, we can clearly see that the interactions of GDNF and ET3 have in vivo differs in culture conditions and that while GDNF is consistent with its characteristics as a proliferation factor, both factors strongly stimulate neuronal or glial phenotype. The effect of Et3 from these results is inconclusive regarding a blocking on differentiation feature of GDNF, but it is clear about its proliferator ability. Based on this it would be possible to control the proliferation of progenitors to some critical point and then induce any of the phenotypes of interest depending on the needs of transplant at a given time.
In our conditions, it is clear that GDNF keeps a powerful proliferator effect, but this effect is not unique on the neuronal phenotype but also on the glial one and perhaps more intense. ET3 also shows a differentiating effect on glia, although perhaps its most significant action in this model is its ability to stop the proliferation, no the differentiation induced by GDNF. Therefore, a strategy that seeks to control cell density without affecting the balance of the neuronal and glial populations should be considered prior exposure to GDNF and then ET3.
In conclusion, this work provides evidence that ET3 blocks the differentiation but also the proliferation of neural progenitors when its effects precede those of GDNF, however, can promote the glial phenotype when used in parallel or after GDNF stimulus, showing the timing of interaction between these two factors is critical to neural enteric cells development.
This work was supported by a posdoctoral fellowship from CONACYT, Mexico (A. Carreon-Rodriguez).
The author declares no conflict of interest.

©2017 Carreón-Rodríguez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.