Journal of
eISSN: 2475-5540


Research Article Volume 2 Issue 3
1Department of Neuroscience, Uppsala University, Sweden
2Institute of Cell Biophysics, Russian Academy of Sciences, Russia
3Nanologica AB, Sweden
4Macquarie University, Australia
Correspondence: Elena N Kozlova, Uppsala University Biomedical Center, Box 593 751 24 Uppsala, Sweden, Tel +46 18 471 4968, Fax +46 18 51 15 40
Received: January 28, 2017 | Published: March 21, 2017
Citation: Ivert P, Otterbeck A, Panchenko M, et al. The Effect of Mesoporous Silica Particles on Stem Cell Differentiation. J Stem Cell Res Ther. 2017;2(3):73-78. DOI: 10.15406/jsrt.2017.02.00063
Stem cell transplantation is an attractive strategy to counteract the progression of neurodegenerative disorders and to replace lost neuronal cells. Despite successful generation of neuronal cell types in vitro, stem cell technology typically fails when applied in vivo. One of the reasons is lack of control over the differentiation process of transplanted stem cells. Presently used differentiation protocols make use of external growth factors to guide differentiation of stem cells into the desired cell types. These protocols work for differentiation of stem cells in vitro, but are not readily transferable to in vivo application, where how to deliver these factors and control their dosage are major challenges. We recently showed that mesoporous silica particles (MSPs) provide a useful system to transport and deliver a chosen mixture of biomimetic growth factors over a given period of time, allowing precise control of stem cell differentiation, independent of the local recipient surroundings. However, the effect of unloaded MSPs on the differentiation of stem cells and on immune cell response in vivo has not been examined. Here we show that unloaded MSPs, as prepared here, have no adverse effect on the formation of neurospheres from boundary cap neural crest stem cells, and that neuronal differentiation in vitro actually is enhanced. After in vivo implantation MSPs are present during 1 month after implantation, i.e. well within the time period when mimetics are expected to be released from the particles. After implantation to injured dorsal roots, but not after injection into the intact spinal cord, some MSPs were taken up by microglia/macrophages. These findings suggest that MSPs, used in this study, can be safely used for the delivery of trophic factors in vitro without effecting the early differentiation of cultured stem cells as well as in vivo for the delivery of factors for several weeks without being taken up by local immune cells.
Keywords: nanoparticles, stem cells, mesoporous silica, neuron differentiation, stem cell transplantation
bNCSC, boundary cap neural crest stem cell; DIFF, differentiation medium; eGFP, enhanced green fluorescent protein; GFAP, glial fibrillary acidic protein; Iba-1, ionized calcium binding adaptor molecule 1; MSP, mesoporous silica particle; PBS, phosphate buffered saline; TRITC, tetramethylrhodamine-5-(and 6)-isothiocyanate; Vol, volume; Wt, weight
Mesoporous silica particles (MSPs) are characterized by ordered porosity, sharp pore size distributions, high internal surface areas, and large pore volumes.1,2 Control over these structural parameters makes them an ideal candidate for drug encapsulation, perfectly suited to uptake and carry large amounts of drugs that then get released with constant concentration.3–5 The release of the actives can be diffusion controlled or may be triggered by a change in media temperature or pH.6 Creation of simultaneous release profiles is possible by using different pore structures (e. g., 2D hexagonal and 3D cubic) that enable a continuous discharge of a fine tuned mixture of active drugs over a given period of time.
MSPs have already shown potential for life science applications over traditional polymer based delivery systems. They offer increased bioavailability, biocompatibility, controlled and targeted release, reduced drug-drug interactions, the potential to deliver both lipophilic and hydrophilic drugs simultaneously, and the ability to customize release profiles for a combination of drugs. Moreover, nanoporous MSPs hold the potential to be not only a very efficient but also a cost effective drug delivery system.
We previously showed that MSPs loaded with growth factor mimetics promote survival and differentiation of co-implanted neural stem cells,7 indicating that the MSP delivery system can serve as a valuable tool for controlling differentiation of transplanted stem cells. Toxicological data from in vitro and in vivo studies suggest that unloaded MSPs have no observable harmful effects and are well tolerated.8–10 However the possible influence of MSPs, used in our studies, on stem cell differentiation and on the non-neuronal response in the central nervous system has not been examined previously. Here we tested the effect of unloaded MSPs on in vitro differentiation of boundary cap neural crest stem cells (bNCSCs), a source of stem cells with remarkable therapeutic potential, and also analyzed the fate of MSPs at different time points after implantation into the spinal cord and on its surface.
bNCSCs are neural crest derivatives that populate the entry/exit points of spinal roots during embryonic development,11,12 participate in cell migration and axon growth control at the spinal root-spinal cord interface,13–15 contribute Schwann cells of spinal roots, nociceptive and thermoceptive neurons11,12 and satellite cells to dorsal root ganglia (DRGs),11 as well as terminal Schwann cells in the skin.16 bNCSCs are able to generate central glial and neuronal cells in vitro and after transplantation in vivo.17–19 During their differentiation to neurons and glia, bNCSCs also have a unique ability to induce proliferation of insulin producing beta cells and to increase beta cell survival and improve their function in co-culture20,21 and co-transplantation22 experiments. However, undifferentiated bNCSCs or their differentiated derivatives do not have beneficial effects on beta cell proliferation and function.20,21 Thus, controlling the differentiation of transplanted bNCSCs is crucial for optimal exploitation of their beneficial properties.
Here we present data on the effect of MSPs on survival and differentiation of bNCSCs in vitro, and on the immune cell response to MSPs implanted to the spinal cord or placed on its surface.
Animals
As recipients for transplantation we used adult male NMRI mice (25-35g body weight; Möllegaard, Denmark). All animal experiments were approved by the Regional Ethical Committee for Animal Experimentation, Uppsala, as required by Swedish Legislation and in accordance with European Union Directives.
Mesoporous silica particles
Mesoporous silica nanoparticles (AMS-6, 3d-cubic porous structure, 300nm spherical particle size and approximately 4.0nm cylindrical pores) were prepared as previously described.23,24 The particles were then conjugated with tetramethylrhodamine-5-(and 6)-isothiocyanate (TRITC; 5 mg; Sigma-Aldrich, MO, USA), achieved by the reaction of amine-functionalized mesoporous extracted materials with the fluorochrome under alkaline conditions in order to produce the imminothioester bond.25
Boundary cap neural crest stem cell (bNCSC) cultures
bNCSC cultures were prepared from E11.5 day old eGFP mouse embryos, as previously described.11 Briefly, DRGs with attached roots were collected and subsequently dissociated by enzymatic treatment using Collagenase/Dispase (1mg/ml, Roche Diagnostics) and DNase (0.5mg/ml, Sigma-Aldrich) at 37°C for 30 minutes. Collected DRGs were rinsed 3 times in phosphate buffered saline (PBS) and placed in DMEM/F12 medium (Invitrogen, Carlsbad, CA, USA), supplemented with N-2 (Invitrogen) (N-2 medium). After mechanical dissociation the cells were plated 1-2 x 105 cells per well in a 24-well plate in propagation medium consisting of DMEM/F12 medium supplemented with N-2, B-27 (Invitrogen) with addition of epidermal growth factor (20ng/mL) (R&D Systems, Minneapolis MN, USA) and basic fibroblast growth factor (20ng/mL) (R&D Systems). After 12 hours all non-adherent cells were removed and fresh propagation medium was added. Formation of neurospheres occurred after 3 weeks of culture. Half of the media volume was replaced every second day, TrypLE Express (1X) (Invitrogen) was used for single cell suspension preparation. Suspension of bNCSCs where plated alone or together with 10ng of TRITC-labeled MSPs and cultured for 5 days in propagation medium for neurosphere formation. Fluorescent images were taken daily in an inverted fluorescence microscope to analyze the distribution of MSPs (red) and bNCSCs (green).
Differentiation assay
For in vitro differentiation assay neurospheres or single bNCSC suspension was used. Neurospheres or dissociated cells were placed alone (control group) or incubated together with MSPs in a 1:1 ratio during one hour with subsequent seeding at a density of 1x105 cells per well in medium. bNCSCs were seeded on poly-d-lysine (0.1mg/mL; Sigma) and laminin-coated (0.1mg/mL; Sigma) circular glass cover slips (12mm in diameter) in 4-well plates (Nunc) in 500µL differentiation medium (DIFF) consisted of 50% DMEM/F12 medium and 50% neurobasal (Invitrogen) supplemented with B27, N2 and NEAA. After 3 days the cells were fixed with 4% formaldehyde (vol/vol) and 14% saturated picric acid (wt/vol) in PBS during 10 minutes and then rinsed 3 times for 10 minutes in PBS before application of antibodies. The MSP powder was diluted in DIFF medium and added to the cultures in a concentration of 10ng/ml.
Surgery
Six adult nu/nu mice (ca 30g body weight) were subjected to dorsal root avulsion MSP implantation, and 6 mice were subjected to injection of MSPs into the spinal cord. On the day of the experiment MSP powder was spinned down and diluted with PBS to a concentration of 1µg/µl. Animals subjected to dorsal root avulsion injury were anesthetized with a mixture of ketamine, xylazine and acepromazine (100, 20, and 3µg/g body weight, respectively) intraperitoneally. The left lumber 3-6 dorsal roots were exposed via a partial laminectomy and durectomy, pulled away from the spinal cord and re-attached. MSPs were placed on the top of the re-attached dorsal roots on the surface of the spinal cord. The wound was closed in layers.
Animals subjected to intraspinal injections were performed using a protocol earlier explained.26 The animals were anesthetized by spontaneous inhalation of isoflurane. After dissection of the back muscles, the laminae of the cervical vertebrae were exposed and a partial laminectomy was made of cervical vertebrae 3 to 5. Four µL (3 injections) of MSP solution were injected into the left ventral horn using a Hamilton syringe with a metal needle (26 gauge) attached to a stereotactic frame and connected to an infusion pump (KD Scientific Legato 130).
Two weeks or one month after surgery 3 animals from both experimental groups were re-anesthetized with an intraperitoneal injection of a mixture of ketamine, xylazine and acepromazine, and perfused via the left ventricle with warm saline solution (~38°C) followed by a cold (~4°C) fixative solution consisting of 4% formaldehyde (vol/vol), 14% saturated picric acid (wt/vol) in phosphate buffered saline (PBS; pH 7.35-7.45). The relevant part of the spinal cord was removed, placed in fixative solution for 4 hours, and thereafter cryoprotected overnight in PBS containing 15% sucrose. The following day the tissue was placed in TissueTech™ and frozen in liquid nitrogen. Transverse sections (14µl) were cut on a cryostat, collected on SuperFrost™ Plus slides (Menzel-Gläser, Braunschweig, Germany, http://w ww.menzel.de), and processed for immunohistochemistry and microscopic analysis as described below.
Immunohistochemistry
Coverslips or cryosections of tissue samples were incubated during one hour at room temperature with blocking solution containing 1% bovine serum albumin, 0.3% Triton X-100, and 0.1% NaN3 in PBS and then incubated overnight at 4°C with primary antibodies to βIII-tubulin for neuronal labeling and glial fibrillary acidic protein (GFAP) for glial cell labeling. Sections harvested from spinal cord (including avulsed dorsal roots) were also labeled with antibody Iba-1, a marker for microglia/macrophages. The next day the coverslips were washed with PBS and incubated with secondary antibodies for one hour. Nuclei were stained with ProLong Gold antifade reagent with Hoechst nuclear stain (Table 1).
Antigen/label |
Host |
Source |
Dilution |
β-III-tubulin (primary) |
Mouse |
Invitrogen |
0.3888889 |
GFAP (primary) |
Rabbit |
DAKO |
0.3888889 |
Iba-1 (primary) |
Rabbit |
Nordic Biolabs |
0.1805556 |
Cy3 (secondary) |
Donkey anti-mouse |
Jackson Immuno Reasearch |
0.3888889 |
Alexa 647 (secondary) |
Donkey anti-rabbit |
Invitrogen |
0.7361111 |
Table 1 Antibodies used for immunohistochemistry
Microscopy
For examination and image capturing a Zeiss LSM 780 Meta laser scanning confocal microscope was used with a 40x objective. Random regions from coverslips were photographed, from three independent experiments for both groups (cells cultured alone or with MSPs). From cryosections the sections were taken for analysis where TRITC-labeled particles were detected.
Quantification of cells in vitro
The number of cells was quantified by counting Hoechst labeled nuclei using the manual cell counter in the software ImageJ (National Institutes of Health, MD, US). To count the number of neurons, Hoechst and βIII-tubulin labeling were used. Only cells displaying a clear neuronal morphology with a cytoplasm around the Hoechst stained nucleus and clear neurite extensions were counted. The number of glial cells was counted using Hoechst and GFAP labeling and all cells displaying clear cell morphology were counted as glial cells.
Differentiation of neurons was assessed in analogy with a previously established procedure.27 In brief, images (at least 5 images from each culture; the cultures were repeated at least 3 times) were placed to Image J program, which calculates the proportion of neurite intersections with the horizontal lines in the program sheath in relation to the number the cell bodies in the image. This method reflects the level of neuronal differentiation, since the more differentiated a neuron is, the longer axonal extensions it has. Significance was detected with a standard t-test between MSP treated and untreated cultures.
In vitro
Neurosphere formation: After thawing, bNCSC neurospheres were dissociated to single cells and put into a differentiation assay to compare the differentiation potential of neurospheres formed in the presence of unloaded mesoporous silica particles and without particles. The GFP expression in the bNCSCs and the TRITC-conjugation of MSPs allowed us to analyze the distribution of both cells and particles in live cultures. In 24 hours small clusters of bNCSCs with attached MSPs were observed (Figure 1). As time progressed, the clusters of cells became increasingly larger in size and particles were mostly found in the center of the neurospheres on day 2 (Figure 1), and were distributed randomly inside the neurospheres on day 3 (Figure 1). The neurospheres formed in the presence of MSPs were similar to the neurospheres, which were formed in parallel without MSPs (Figure 1).
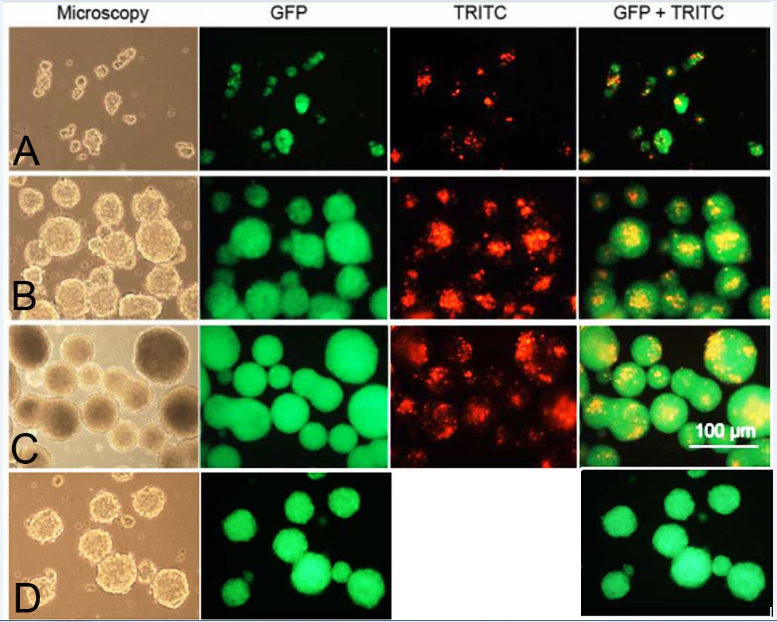
Figure 1 Live images of formation of bNCSC neurospheres from dissociated single bNCSCs cultured together with TRITC-labelled MSPs after 1(A), 2(B) and 3(C) days in propagation medium and without MSP at day 3(D). Scale bar: 100μm.
Differentiation assay: After neurospheres were formed, they were dissociated to single cells and put into a differentiation assay to compare the differentiation potential of neurospheres formed in the presence of unloaded MSPs and without particles.
After 3 days, the mean number of cells in the control group was 343 in contrast to the particle group that was 124. Cultures of the control group showed a significantly higher cell number than cultures of the particle group (Figure 2A, p<0.001). At the same time the mean number of neurons in the control group was markedly higher than in treated group (197 in control and 67 in particle group, p < 0.001, Figure 2B). The mean number of glial cells in the control group and in the particle group was similar (25 in control group and 23 in MSP group, p=0.637, Figure 2C).
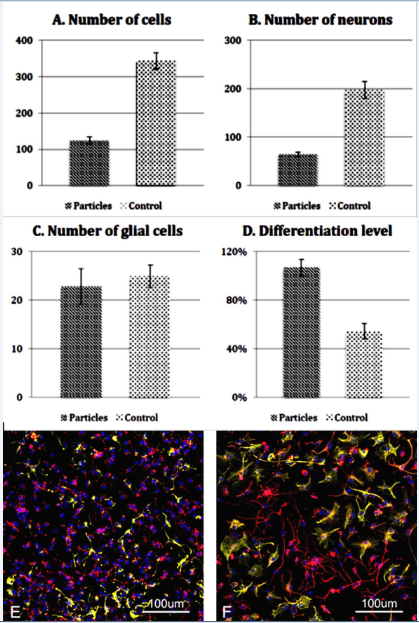
Figure 2 Quantitative analysis of MSP treated (Particles) and untreated (Control) bNCSCs after 3 days in culture. Total number of cells (A), number of neurons (B), number of glial cells (C), and level of neuronal differentiation (D). Data show means ± SEM. E-F: Images of untreated (E) and MSP treated (F) bNCSC cultures labeled with the neuronal marker βIII-tubulin (red), the glial marker GFAP (green), and the nuclear marker Hoechst (blue). Scale bar: 100μm.
When cells were labeled with βIII-tubulin antibodies (neuronal marker), neurons in the MSP group displayed more differentiated cells, as evidenced by more and longer axon arborizations. In the control group (Figure 2D & 2E) neurons were smaller and less differentiated (Figure 2F). This was confirmed using a procedure for quantification of neurite outgrowth (see Material and Methods). The differentiation level in the control group was 54%, whereas the differentiation level in the particle group was 107% (Figure 2D; p < 0.001).
In vivo
MSPs were placed either on the surface of avulsed spinal cord (6 animals) or injected into the intact spinal cord (6 animals). Three animals from each group were analyzed 2 weeks and 1 month, respectively, after MSP administration.
TRITC-labeled MSPs implanted to the avulsed and re-attached dorsal roots were detected on the surface of the spinal cord (Figure 3), within the dorsal roots as well as inside the spinal cord (Figure 3, arrowheads), both at 14 days and at one month after implantation (not shown). No particles were detected in the DRGs (not shown). Some of the MSPs particles were closely associated with Iba-1 immunoreactivity, and seemed to be located inside the cells, suggesting that they had been taken up by microglia/macrophages (Figure 3, insert). The overall microglia/macrophage reaction was similar in MSP treated and untreated animals.
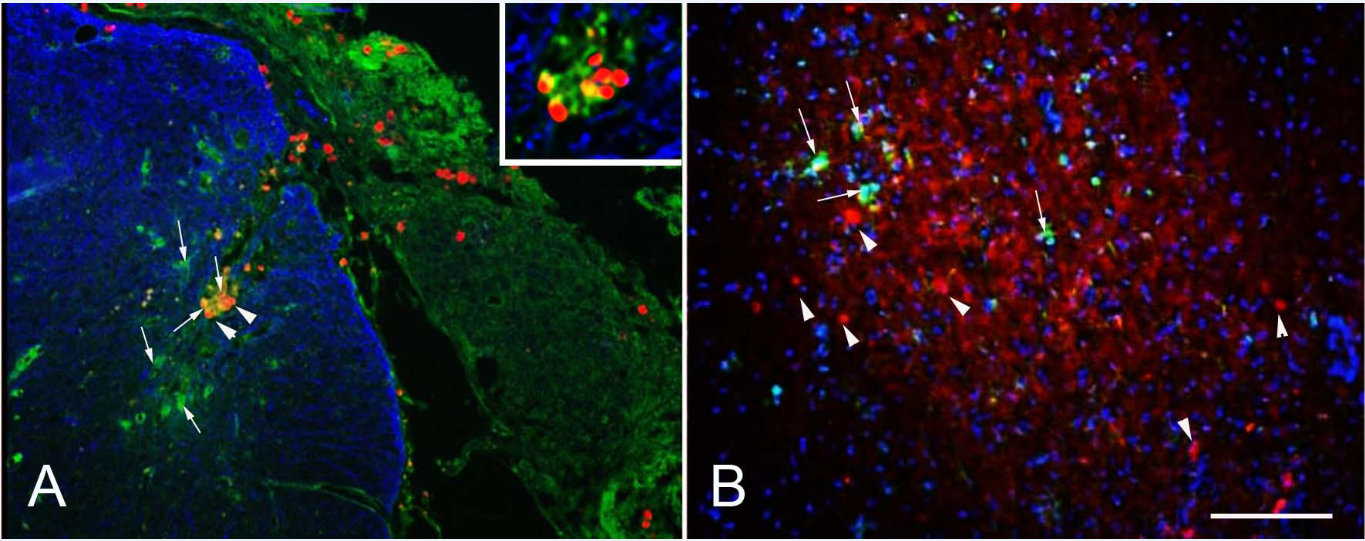
Figure 3 After dorsal root avulsion implanted MSPs (red) were on the surface of the spinal cord (A) as well as inside the spinal cord (arrowheads), where some of them were closely associated with microglia/macrophages (Iba-1, green, arrows). The astrocytic staining GFAP (blue) shows the boundary between the central (blue) and peripheral nervous system. After injection into the spinal cord the MSPs (arrowheads) were not associated with Iba-1 positive microglia/macrophages (green, arrows). Scale bar: 100µm.
When MSPs were injected into the ventral horn of the spinal cord, we detected no association of MSPs with microglia marker Iba-1, suggesting that they did not induce microglia/macrophage activation, which was mostly possible due to the injection procedure to the spinal cord.
The goal of this study was to examine the effect of unloaded MSPs in vitro on differentiation of bNCSCs and to investigate their presence in vivo after implantation. We found that MSPs have no adverse effect on the differentiation of stem cells in vitro, when we grew bNCSC neurospheres. We observed particles in close proximity to the newly formed neurospheres, and their penetration inside the neurospheres, but we did not detect any abnormalities in neurosphere formation. When particles were added to dissociated neurospheres placed in differentiation assay, we observed a lower number of cells in MSP treated compared to untreated cultures, and the proportion of neurons was increased in untreated cultures. However, neuron differentiation, assessed as degree of axonal extensions, was clearly more advanced in MSP treated compared to untreated cultures. These data suggest that the presence of unloaded MSPs promoted neuronal differentiation of stem cells, at the expense of overall cell survival. Another explanation to the reduced number of cells in treated cultures would be the induction of cells death by MSPs. However increased neuronal differentiation and equal number of glial cells in both cultures favor the suggestion that MSPs facilitate/induce neuronal differentiation. During development neurogenesis starts prior to gliogenesis, but there is a switch point after which both these processes occur in parallel. Thus the presence of MSPs in the culture may speed up the time of the switch point.
It was suggested that the particles after cytoplasmic penetration might exert an effect on intracellular processes, including cell viability.28,29 The particles, that were shown to be located close to the cells, might have an effect on the neurite formation and/or the length of neurites. It can also be argued that the enhanced differentiation could be a result of the reduced number of cells, creating an increased intracellular space and leading to a more favorable environment for differentiation. Another explanation can be that the increased proportion of glial cells in MSP treated cultures is beneficial for neural differentiation.
In vivoimplantation resulted in distinctly different distribution of MSPs depending on whether the implant site had been subject to injury or was intact at the time of implantation. MSPs implanted to the injured dorsal roots were to some degree taken up by microglia/macrophages. Still, a substantial proportion of MSPs remained extracellularly for the entire one month post-implantation period. Following injection into the intact spinal cord, MSPs were not observed in microglial cells. These finding strongly indicate that factor loaded MSPs are able to serve as a viable source for in vivo delivery of growth factor mimetics or other bioactive agents during at least one month, a period which is sufficient to induce appropriate differentiation of transplanted stem cells.7
Our data show that MSPs do not have adverse effect on the formation of stem cell neurospheres and in differentiation assay induce neuronal differentiation. These data show that MSPs can be used as a vehicle for the delivery of trophic factors in vitro facilitating culture work and reducing risk of contamination. After implantation to the avulsed spinal cord or injection into the spinal cord, the MSPs located in the area of administration during one month what is sufficient for delivery of loaded trophic factors to the co-implanted stem cells or damaged cells/tissues.
The study was supported by the Swedish Research Council (Project No 20716), the Swedish Research Council under the frame of EuroNanoMed-II, Stiftelsen Olle Engkvist Byggmästare, and Signhild Engkvists Stiftelse.
The author declares no conflict of interest.

©2017 Ivert, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.