Journal of
eISSN: 2475-5540


Research Article Volume 2 Issue 1
1Department of Pathology, Johns Hopkins University, USA
2Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, USA
Correspondence: Pampee Young, Department of Pathology, Tennessee Valley Veterans Affairs Hospital, 1161 21st Avenue South, MCN C3321A, Nashville, TN, USA, Tel (615) 9361098, Fax (615) 3437023
Received: September 10, 2016 | Published: January 18, 2017
Citation: Gehrie EA, Young PP. A2 erythrocytes lack a antigen modified glycoproteins which are present in A1 erythrocytes. J Stem Cell Res Ther.2017;2(1):24-28. DOI: 10.15406/jsrt.2017.02.00053
Background and objectives: There is considerable disagreement in the literature regarding the nature of differences underlying subgroups of blood group A. The purpose of this study is to further investigate possible qualitative and quantitative variations between A1 and A2
Materials and methods: Erythrocytes from type A blood donors were tested for hemagglutination with A and B monoclonal antibodies and the A1 lectin, Dolichos biflorus. A2 subgroup was assigned to those A erythrocytes that did not react with Dolichos biflorus but did react strongly with A antibody. Once A1 and A2 cells were thus identified, variation in A antigen expression was assessed by flow cytometry and western blot.
Results: Flow cytometry revealed that A2 cells express less A antigen than A1 cells, but the extent of the difference was less than expected and decreased as the dilution of the A antibody increased. However, when A1 and A2 erythrocytes were studied by western blot, A1 erythrocytes yielded dramatic protein bands, which A2 erythrocytes failed to demonstrate.
Conclusion: Only A1 erythrocytes expressed antigen identified by western blot. This dramatic qualitative difference between A1 and A2 cells seems to be more substantial than the small quantitative differences detected by flow cytometry.
Keywords: Blood groups; Immuno hematology; RBC antigens and antibodies; Flow cytometry
The major blood group antigens A and B are sugars that are expressed on red blood cells, on organ endothelia, and in the body fluids of most individuals.1–3 The biological significance of blood group in nature is unknown, although the distribution of blood groups throughout the world may be explained in part by susceptibility to various diseases.4–5 Blood group is a major consideration in transfusion medicine and organ transplantation because ABO incompatible transfusions and allografts may precipitate catastrophic hemolysis or graft thrombosis resulting in patient death.6–8
Blood group A is defined by the presence of the sugar n-acetyl galactosamine (and the absence of galactose) on the terminal galactose of glycolipid and glycoprotein structures attached to the erythrocyte surface.9 Approximately 41.7% of individuals of European descent are blood group A.10 Almost 80% of European-descended blood group A individuals are subcategorized as A1, which is defined by hemagglutination with the lectin of Dolichos biflorus. In contrast, approximately 22% of blood group A individuals of European ancestry are subgroup A2, making the A2 subgroup the second most common A subgroup (after A1).10 It is important to note that other ethnicities have different blood group distributions; for example, the A2 subtype is rare (<1%) in Japan.11 Other than A1 and A2, the remaining A subgroups, such as A3, Ax, Aint, and Am (to name a few) are relatively rare and are usually detected via a weak or mixed field hemagglutination with A antibody.9 Approximately 75-95% of blood group antigen determinants are bound to protein backbone structures, with the remaining antigen expressed on lipid backbones.9,12
The nature of the mechanism underlying the difference between A1 and A2 erythrocytes has been a controversy for decades, with the literature divided among studies promoting a qualitative mechanism,13–18 studies demonstrating a quantitative mechanism,19–22 or studies advocating for both qualitative and quantitative differences.23 It has been prominently reported that group A2 erythrocytes express approximately 75% less A antigen on their surface relative to A1 erythrocytes.20–22 However, because A2 erythrocytes are believed to express a relatively large number of A antigen sites (~250,000 antigen sites per cell),20 it may be that anti-A1 is formed for a reason other than the A1 antigen being recognized as “foreign”. An alternative hypothesis is that a qualitative difference in the structure of some A antigen expressing proteins or lipids may underlie the immunology of A1 antibody production. Such a qualitative difference may also explain the reduced immunogenicity of A2 solid organ allografts relative to A1 in the context of ABO incompatible transplantation.
In order to further investigate the existence of qualitative and quantitative differences in A antigen expression, we determined expression of A antigen on A1 and A2 erythrocytes via several methodologies: flow cytometry and western blot.
Erythrocytes
Erythrocytes were obtained with IRB approval from tubing segments from 87 blood group A red blood cell donor units in inventory at the Vanderbilt University Medical Center Blood Bank. All donors segments were tested via hemagglutination using commercially available A and B antibodies (Immucor, Norcross, GA) to confirm their blood grouping as group A. Each segment was also tested with Dolichos biflorus, the A1 lectin, to help to determine A1 versus non-A1 subgroup (Immucor, Norcross, GA). Donors were not pre-selected for A1 or A2 subgroup.
Serial dilution hemagglutination (tube) assay
A1 and A2 cells were identified based on hemagglutination (or lack thereof) with Dolichos biflorus and strong hemagglutination reactions with monoclonal A antibody (Immucor, Norcross, GA). To test for subtle differences in antigen expression between A1 and A2 cells, serial dilutions of A antibody (Immucor, Norcross, GA) were prepared with buffered PBS as the diluent. One drop of 2% erythrocyte suspension in buffered PBS was added to 2 drops of diluted antibody in a 10mm diameter glass test tube. The suspensions were centrifuged immediately for 20 seconds at 3480rpm in a serologic centrifuge (BD Biosciences, Franklin Lakes, NJ) at room temperature and then assessed for hemagglutination.
Flow cytometry
Red cells from A1 and A2 donor segments identified from hemagglutination testing (above) were washed three times in flow cytometry buffer. The washed cells were counted in a hemocytometer (Hausser Scientific, Horsham, PA). A volume corresponding to 5x106 erythrocytes from each donor was suspended in 100mcL of flow cytometry staining buffer and incubated at 4°C for 30 minutes with A antibody (Immucor, Norcross, GA) diluted 1:20, 1:40, 1:80 or 1:160 in PBS. The A antibody was subsequently removed with 3 sequential washing and centrifugation cycles (at 3,000xg for 3 minutes at 4°C). The washed cells were re-suspended in approximately 100mcL of flow cytometry staining buffer and incubated with 30mcg of R-Phycoerythrin-conjugated mouse antibody (Jackson Immuno Research Laboratories, West Grove, PA) at 4°C for 30 minutes in the dark. After incubation, the cells were washed 3 times in flow cytometry staining buffer and then analyzed on an LSR II flow cytometer (BD Biosciences, San Diego, CA). Histograms were constructed using FlowJo software (Treestar, Ashland, OR).
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) western blot
A1 and A2 erythrocytes (as determined above) were washed in PBS and lysed with five consecutive centrifugation (at 5,000xg for 5 minutes) re-suspension cycles in a hypotonic solution of 5mM NaPO4 supplemented with protease inhibitors (Roche, Indianapolis, IN). The resulting pearly white erythrocyte “ghosts” were washed a final time in PBS and solubilized in RIPA buffer. The protein concentration from each donor was determined by the bicinchoninic acid assay (Pierce Protein Research Products, Rockford, IL). After quantification, equal quantities of protein (either 10mcg or 20mcg) from each donor were loaded into a 12% polyacrylamide gel. After electrophoresis for 2 hours at 110V, the gel was transferred for approximately 16 hours onto a nitrocellulose membrane at 4°C at 30V. Next, the nitrocellulose membranes were blocked with 5% milk in TBS-T and probed for A antigen using a 1:1000 dilution of monoclonal A antibody (Immucor, Norcross, GA) for 2 hours at room temperature. After washing the membrane for 30 minutes in TBS-T, it was incubated with a horseradish peroxidase conjugated secondary mouse antibody (Southern Biotech, Birmingham AL) at room temperature for 1hour. After washing, the membranes were treated with horseradish peroxidase chemiluminescent substrate (Millipore, Billerica MA) and exposed to film in the dark for 5 to 25 minutes. The membranes were subsequently washed gently until the A antigen signal was removed. The membranes were then probed for beta actin (Sigma, St. Louis, MO) for 2 hours at room temperature, washed, probed with secondary mouse antibody for one hour (Southern Biotech, Birmingham, AL) and re-exposed.
Statistics
Unpaired t-tests were performed to compare the mean fluorescence intensity (MFI) of A1 versus A2 red blood cells. Two tailed p values were calculated and any p value <0.05 was considered statistically significant. Analysis was performed using GraphPad Prism version 7 (Graphpad Software Inc, La Jolla, CA).
Hemagglutination (tube) assay
All cells, regardless of their reaction with Dolichos Biflorus, reacted strongly with no mixed field agglutination with A antibody. In general, the monoclonal antibody agglutinated all cells at 4+ strength until it was diluted 1:16 or greater. At dilutions greater than 1:16, both A1 and A2 cells experienced a gradual decline in hemagglutination strength until all group A cells were negative at the 1:1024 dilution (Table 1). Thus, all of the non-A1 cells used in our study were classified as subgroup A2.
Specimen |
Dolichos Biflorus |
1:01 |
1:02 |
1:04 |
1:08 |
1:16 |
1:32 |
1:64 |
1:128 |
1:256 |
1:512 |
1:1024 |
A |
- |
4+ |
4+ |
4+ |
4+ |
4+ |
3+ |
2+ |
2.5+ |
2+ |
1+ |
0 |
B |
- |
4+ |
4+ |
4+ |
4+ |
3.5+ |
3+ |
2+ |
2+ |
1.5+ |
1+ |
0 |
C |
- |
4+ |
4+ |
4+ |
4+ |
4+ |
3+ |
2+ |
2.5+ |
2+ |
1+ |
0 |
D |
+ |
4+ |
4+ |
4+ |
4+ |
4+ |
3+ |
3+ |
3+ |
1.5+ |
1.5+ |
0 |
E |
+ |
4+ |
4+ |
4+ |
4+ |
4+ |
3+ |
2+ |
2+ |
2+ |
1.5+ |
0 |
F |
+ |
4+ |
4+ |
4+ |
4+ |
4+ |
3.5+ |
2.5+ |
2+ |
2+ |
1+ |
0 |
Table 1 Serial dilution hemagglutination studies with monoclonal A antibody in A1 versus A2 erythrocytes. Data shown from 6 donors (3 A1 and 3 A2) analyzed together
Flow cytometry
We found that A2 erythrocytes express slightly less A antigen than A1 erythrocytes by flow cytometry, but the extent of the difference was dependent on the concentration of A antibody used in the assay (Figure 1). The difference between A1 and A2 cells was most apparent at the 1:20 dilution (mean A1 MFI=717; mean A2 MFI=445; p=0.0418), and tapered off until it was very slight at best at a dilution of 1:160 (mean A1 MFI=185; mean A2 MFI=187; p=0.8832). Results are summarized in Table 2.
Anti-A Dilution |
A1 MFI (mean) |
A2 MFI (mean) |
p Value |
1:20 |
717 |
445 |
0.0418 |
1:40 |
544 |
414 |
0.1165 |
1:80 |
380 |
260 |
0.0354 |
1:160 |
185 |
187 |
0.8832 |
Table 2 Mean Fluorescence Intensity (MFI) in A1 versus A2 donors at various dilutions of anti-A. n=3 for each dilution tested

Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) western blot
All A1 donors tested generated a wide range of protein bands approximately 37-75kD in size, but A2 donors did not generate any protein bands (Figure 2; results representative of all donors tested). All group A cells, regardless of subgroup, expressed the housekeeping gene beta. When commercially available, known A2 reagent cells were subjected to western blot, the results were indistinguishable from the tested A2 donor erythrocytes (Figure 2A, second lane from right).
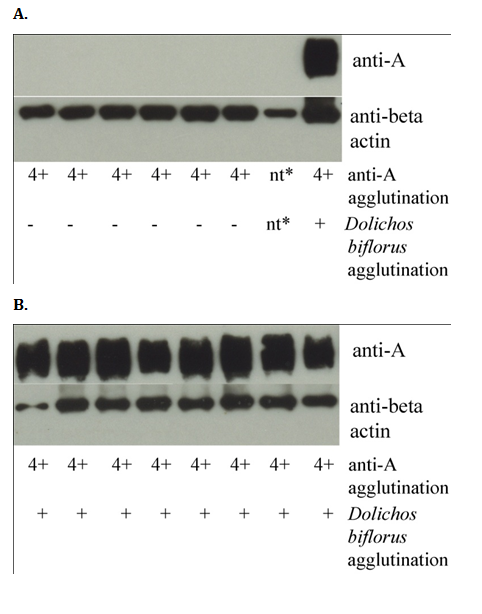
Figure 2 Western blots of erythrocyte membranes probed for A antigen and beta actin. A) A minority of solubilized group A erythrocyte membranes do not yield protein bands when probed with A antibody (middle lane). Cells from these donors did not react with Dolichos biflorus but reacted strongly with A antibody by tube hemagglutination, indicating that they are A2 cells. In contrast, the housekeeping gene beta actin was strongly expressed in all donors. A single A1 donor was run as a positive control in the far right lane. Commercially available A2 reagent cells (second lane from right) were indistinguishable from the donor A2 erythrocytes. B) In contrast, multiple A1 donors yield protein bands when probed with antibody to blood group A antigen (all lanes).
*Not tested; lane loaded with commercially produced reagent A2 cells.
All three of the laboratory modalities that we used to assay for A antigen expression utilized the same commercially produced monoclonal blood group A antibody.24 This antibody is approved by the United States Food and Drug Administration as a blood grouping reagent and is used routinely in our blood bank for clinical specimens.24
Although flow cytometry did detect differences between A1 and A2 erythrocytes, the extent of the differences detected was smaller than what we expected based on the literature (~75% reduction in A2 compared to A1).20–22 Interestingly, as the A antibody was diluted, the difference between A1 and A2 cells became harder to determine (Figure 2). We interpret these results to mean that the differences in the quantity of A antigen expressed on A1 versus A2 cells may be smaller than expected based on previous reports in the literature.20–22
Western blot is a highly sensitive laboratory technique used to detect and quantify proteins. It is rarely employed in clinical settings, although it is used occasionally to provide highly specific, highly sensitive test results (e.g., historically as a confirmation of HIV infection after a positive ELISA).25 If the difference between A1 and A2 cells was primarily quantitative, the western blot of A2 cells would be expected to show a fainter, but identifiable bands, compared to A1 cells. These weaker bands could be taken as evidence of a slow A2 transferase that was biochemically active on all of the same structures as the A1 transferase but was unable to add A determinants as efficiently. However, the complete absence of protein bands on analysis of A2 cells is not consistent with a quantitative difference; rather, it is consistent with a qualitative difference in the structures underlying A antigen determinants on A1 versus A2 cells. Specifically, these results suggest that proteins expressing A antigen on A1 cells do not express A antigen on A2 cells. The presence of beta actin in both A1 and A2 cells serves an internal control that protein was loaded in all experiments. Although we did also detect minor quantitative differences by flow cytometry, the stark contrast between A1 and A2 cells assayed by western blot suggests that a key difference between A1 and A2 cells is qualitative, with discernible but minor differences in A antigen expression as a secondary finding.
Our finding that the difference between A1 and A2 cells may be largely qualitative contradicts a number of studies in the literature that report far greater quantitative differences than the present study.20–22 The most widely cited9,17,18,21,26 study to present evidence of extensive quantitative differences - while extremely elegant and ahead of its time - was published in 1967 and has some important shortcomings.20 Briefly, the study utilized a 25I labeled A antibody that was generated from 2 rabbits injected with human A1 erythrocytes. After purification and radioactive labeling, the A antibody was exposed to formalin-fixed erythrocytes of various known group A subgroups. The number of antigen sites per cell (approximately 1million for subgroup A1 and 250,000 for subgroup A2) was estimated based on serum absorption. However, we hypothesize that the rabbits injected with human A1 erythrocytes may have produced a relatively greater amount of A1 antibody and less A antibody, which would also explain these findings. It is interesting to note that the first A antibody generated from a hybrid myeloma cell line also showed higher avidity for A1 cells than for A2 cells, but subsequent hybridoma formulations react equally with A1 and A2 cells.27. These findings suggest that a falsely depressed determination of A2 cell antigen sites can be calculated using an antibody with reduced A2 selectivity (such as an A1 antibody).
We are certainly not the first group to hypothesize that important qualitative differences distinguish A subgroups. However, previous reports have focused on differences in glycolipids carrying A antigen determinants, rather than proteins. Briefly, thirty years ago, Fujii et al.15 showed that A2 erythrocytes are missing a major glycolipid carrier of A antigen that is found on A1 erythrocytes.15 Five years later, an unrelated group identified a novel glycolipid, known as type 3 chain A, that was believed to express A antigen exclusively by A1 erythrocytes.14 This same group later established that the A2 transferase was far less efficient at converting type 3 or type 4 (globo-H) H structures to type 3 or type 4 A structures relative to the A1 transferase.18 A more recent study elegantly repeated some of the early investigations using many of the same antibodies.17 However, this study found that type 3 glycolipids do express A antigen on A2 erythrocytes, while confirming that type 4 glycolipids carry A antigen determinants on A1 but not A2 cells.17 In contrast to previous studies, the present study is the first of which we are aware that identifies proteins detected by western blot that expresses A antigen on A1 erythrocytes but not A2 erythrocytes. It is interesting to note that glycolipids are only estimated to underlie 4-20% of all A antigen determinants, with protein backbones constituting the remaining 75-95%.9,12 In addition, the type 4 chain A glycolipids - which were confirmed by the most recent major study in the literature as the likely lipid “A1 substance” -17 are very minor contributors to the total glycolipid makeup of erythrocytes.28 This may indicate that a qualitative difference in protein structures expressing A antigen, as reported in the present study, may be especially immunogenic, and possibly more likely to be the antigen responsible for the generation of A1 antibody than a rare lipid based antigen as reported previously. Further research is needed to achieve additional clarity on this subject, however.
The concept that there are substantial differences in H expression between A1 and A2 cells is based on a fundamental pillar of erythrocyte biochemistry: that A and B determinants are generated by the addition of n-acetyl galactosamine or galactose to pre-existing H antigen structures. Assuming that erythrocytes have a finite number of H structures regardless of their grouping (except in rare instances, such as type O Bombay), a more active transferase implies a greater number of A determinants and fewer remaining H antigen sites. Thus, if one assumes that A2 cells express 75% less A antigen than A1 cells, they must also express substantially more H antigen than A1 cells. However, because we report only relatively small difference in A antigen expression overall between A1 and A2 cells, we would expect only a proportional (and also very small) difference in H antigen sites.
We did not employ molecular tests to differentiate A1 and A2 cells for this study. This is because we were able to establish A1 and A2 cells with confidence using monoclonal antibodies and the A1 lectin. In addition, reagent grade A2 cells that we purchased from Immucor provided identical results on western blot as serologically defined A2 cells (Figure 2A, second lane from right). Previous studies comparing A1 and A2 cells have been published based on serological classification of A1 and A2.17
In conclusion, we tested erythrocytes from 87 group A donor segments. All eighty-seven reacted strongly on forward screening with A antibody and sixty-four agglutinated with Dolichos biflorus, indicating that 26.4% of our group A donor population is A2. This is similar to the 22% of total group A individuals of European ancestry that are estimated to be A2 in the medical literature.10 We report that the A1 cells in our cohort express a slightly greater number of total A antigen sites as compared to A2 cells, but the extent of this difference is less striking than the qualitative difference in structures expressing A antigen that we identified by western blot. We believe that further study of this finding could yield important details about blood group antigen immunogenicity, with implications for the fields of transfusion as well as transplantation.
Only A1 erythrocytes expressed protein antigens as identified by western blot. This dramatic qualitative difference between A1 and A2 cells seems to be more substantial than the small quantitative differences detected by flow cytometry. These data suggest that A2 erythrocytes lack certain A antigen-modified glycoproteins and likely harbor mainly glycolipids containing the A antigen.
The authors gratefully acknowledge Shelia Garrett, SBB, Mary S. Johnson, SBB, and Deborah L. Sturdivant, SBB of the Vanderbilt University Medical Center Blood Bank and Justine Liepkalns of Emory University for their generous technical assistance and grant # R01GM11830001.
EAG designed the experiments, performed the experiments and wrote the paper. PPY designed the experiments and wrote the paper.
This research was supported in part by Vanderbilt University Medical Center CTSA Grant # UL1 RR024975-01 from NCRR/NIH (to EAG and PPY) and a Clinical and Translational Enhancement Award from the Vanderbilt University Department of Pathology, Microbiology and Immunology (to EAG and PPY).
The author declares no conflict of interest.

©2017 Gehrie, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.