Journal of
eISSN: 2475-5540


Mini Review Volume 1 Issue 7
1Stem Cell Biology Research Center, Yazd Reproductive Sciences, Institute, Shahid Sadoughi University of Medical Sciences, Iran
2Department of Biology, Ashkezar Islamic Azad University, Iran
3Department of Advanced Medical Sciences and Technologies, Shahid Sadoughi University of Medical Sciences, Iran
Correspondence: Behrouz Aflatoonian, Stem Cell Biology Research Center, Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, Tel 98-0353-824-7085, Fax 98-035-3824-7087
Received: December 11, 2016 | Published: December 30, 2016
Citation: Akyash F, Javidpou M, Nodoushan FS, et al. Human embryonic stem cells derived mesenchymal stem/stromal cells and their use in regenerative medicine. J Stem Cell Res Ther. 2016;1(7):272-274. DOI: 10.15406/jsrt.2016.01.00047
Recently, studies focused on mesenchymal stem/stromal cells (MSCs) as progenitor cells due to their capacity to regenerate mesodermal tissues such as cartilage, adipose and bone. However, there are practical and ethical challenges regarding the isolation of these cells from different sources (mainly bone marrow). The ability to produce MSCs from human embryonic stem cells (hESCs) could be valuable to produce large amounts of genetically identical and modifiable MSCs that can be used to study the biology of MSCs and also, in the future cell therapy applications. In this mini review, we briefly focused on the advances in the differentiation of hESCs into MSCs and application of these cells as novel therapies in tissue engineering and regenerative medicine.
Keywords: cell therapy, human embryonic stem cells, mesenchymal stem/stromal cells, regenerative medicine, tissue engineering
MSCs, mesenchymal stem/stromal cells; BM, bone marrow; ECM, extracellular matrix; hESCs, human embryonic stem cells; hESCs-MSCs, hescs derived mscs; SCID, severe combined immunodeficiency; BM-MSCs, bone marrow derived mscs; AMSCs, adipose derived mscs; NK, natural killer cells; HLA, human leukocyte antigen; CMH, cardiac myosin heavy chain; CTNT, cardiac troponin t; NCX1, sodium-calcium exchanger 1; α-SMA, alpha smooth muscle actin; CTNI, cardiac troponin i; MRCL3, myosin regulatory light chain; SLE, systemic lupus erythematosus; LN, lupus nephritis; IV, intravenously
According to the reports, multipotent mesenchymal stem/stromal cells (MSCs) can be isolated from bone marrow (BM), adipose tissue, umbilical cord, cord blood, amniotic membrane, amniotic fluid and other sources.1 These spindle shape cells characterized with expression of specific surface markers such as CD29, CD44, CD73, CD90, CD105, CD166 and lack of hematopoietic and endothelial markers such as CD34, CD45, CD11b, CD14.2–4 It was demonstrated that MSCs have immunomodulatory properties like avoidance graft rejection due to immune tolerance during transplantation and autoimmunity.3 Moreover, MSCs can differentiate into mesodermal cell lineages including chondrocytes, osteoblasts, and adipocytes.2 Another critical role of MSCs is cytokines, chemokines and extracellular matrix (ECM) proteins secretion to repair of damaged tissues.2,5 Based on the current studies, MSCs identified as favorable candidate in the field of cell therapy and subsequent tissue engineering and regenerative medicine.2,5
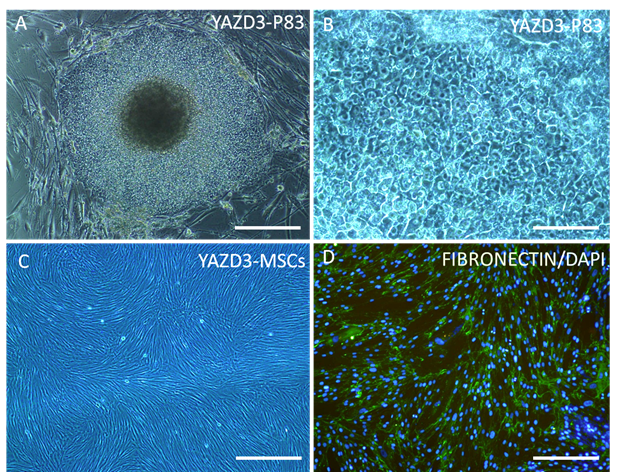
Human embryonic stem cells (hESCs) which are derived from the pre-implantation embryos are identified as pluripotent cells with capacity to differentiate into every adult cell types that derived from three germ layers (ectoderm, mesoderm and endoderm)6,7 and also germ cells.8 Recent studies reported that MSCs can be derived from hESCs9–11 that these differentiated cells expressed MSCs surface markers.2,9 Also, our preliminary data showed hESCs derived MSCs (hESCs-MSCs) with fibroblast- like morphology (Figure 1) expressed CD105 and FIBRONECTIN as MSC specific markers (unpublished data; Figure 1). Karlsson et al.12 used Xeno-free derivation of MSCs from hESCs as an optimal protocol for the first time and reported these cells with MSCs phenotype and differentiation potential into mesodermal tissues do not form teratomas when transplanted under the kidney capsule of severe combined immunodeficiency (SCID) mice.12 Similar to these potential, reports demonstrated that hESCs-MSCs not only were phenotypically similar to bone marrow derived MSCs (BM-MSCs) and adipose derived MSCs (AMSCs), but also, showed higher in vitro proliferation capacity and equivalent differentiation potential into adipocytes and osteocytes.13 Immunomodulatory role of BM-MSCs reported throughout immune cells interaction, such as T and B lymphocytes, natural killer (NK) cells, and dendritic cells.3,14 Human leukocyte antigen (HLA) type I detected on the surface of human MSCs unlike HLA type II which do not express on the cell surface.15 These immunomodulatory characteristics confirmed in hESCs-MSCs which identified with HLA type I expression but not HLA type II and CD80 and CD86 as costimulatory molecules.16 On the other hand, reports showed MSCs transplantation do not need HLA-matching, indeed, these cells can be used for number of recipients regardless donor's HLA typing.2 Hence, hESCs-MSCs can be used as a suitable source for future cell therapy clinical applications and stem cell banking.
hESCs - MSCs and regenerative medicine
Hwang et al.17 created articular damage in the rat patellofemoral cartilage of the knee joint and transplanted hESCs-MSCs into defect area, three days after cells culture. Their reports demonstrated direct contribution of the cells in the cartilage repair and histological evaluation showed normal smooth appearance of treated cartilage in comparison with surrounding cartilage.17 Similarly, Laurila et al.18 transplanted hESCs-MSCs and human BM-MSCs into rat lower extremity ischemic model. They evaluated distribution and physiological response of transplanted cells after xenografts. They investigated that the small number of alive cells were remained 24h after transplantation into ischemic region. Moreover, despite the low rate of hESCs-MSCs and BM-MSCs, they have similar effect for enhancement of cells proliferation, vessels formation and subsequently angiogenesis due to growth factors secretion from recipient’s tissues.18 Another study evaluated differentiation potential of hESCs-MSCs into cardiomyocytes like cells. At first, they confirmed similarity of the differentiation ability of hESCs-MSCs and BM-MSCs into osteocytes and adipocytes. Then it was shown that differentiated hESCs-MSCs, express specific cardiac markers including, cardiac myosin heavy chain (CMH), cardiac Troponin T (CTNT), Homeobox protein NKX2.5, sodium-calcium exchanger (NCX1), alpha smooth muscle actin (α-SMA), cardiac Troponin I (CTNI) and myosin regulatory light chain (MRCL3), However, these cell did not create contractile cells.19 In the field of autoimmune diseases, Thiel et al.20 used hESCs-MSCs for systemic lupus erythematosus (SLE)/lupus nephritis (LN) treatment in the mice model. They demonstrated after intravenously (IV) injection of the derivate cells, preserved kidney function and cured the sings of disease such as reduction of proteinuria and serum creatinine levels. It could be postulated, these cell populations due to Immunomodulatory role via reduction of inflammatory cytokine levels and increase of regulatory T cells perform this function.20
Derivation of MSCs from hESCs provides new insight in the field of cell therapy and regenerative medicine for large number of patients. In the future, hESCs-MSCs can be used in the tissue engineering of the bone or cartilage for patients with osteogenesis and chondorgensis disorders. Even, using the direct transplantation of hESCs-MSCs into the defect site, can differentiate them into bone or cartilage according to the environmental condition and as a result repair the damaged area. Moreover, migration of these cells toward injured or inflamed sites helps for tissue regeneration due to cytokines and growth factors secretion. It should be noted that using of hESCs-MSCs as immunomodulator without any HLA matching in transplantation procedure facilitate their entrance into clinical cell therapy applications (Table 1).
Abbreviations |
|
Mesenchymal stem/stromal cells |
MSCs |
Bone marrow |
BM |
Extracellular matrix |
ECM |
Human embryonic stem cells |
hESCs |
hESCs derived MSCs |
hESCs-MSCs |
Severe combined immunodeficiency |
SCID |
Bone marrow derived MSCs |
BM-MSCs |
Adipose derived MSCs |
AMSCs |
Natural killer cells |
NK |
Human leukocyte antigen |
HLA |
Cardiac myosin heavy chain |
CMH |
Cardiac Troponin T |
CTNT |
Sodium-calcium exchanger |
NCX1 |
Alpha smooth muscle actin |
α-SMA |
Cardiac Troponin I |
CTNI |
Myosin regulatory light chain |
MRCL3 |
Systemic lupus erythematosus |
SLE |
Lupus nephritis |
LN |
Intravenously |
IV |
Table of Abbreviations
None.
The author declares no conflict of interest.

©2016 Akyash, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.