Journal of
eISSN: 2475-5540


Mini Review Volume 1 Issue 5
1Department of Biochemistry, Jamia Hamdard Deemed University, India
2Bioinformatics Infrastructure Facility, Jamia Hamdard Deemed University, India
3Emory University, USA
4Department of Animal Nutrition, Firat University, Turkey
Correspondence: Shakir Ali, Department of Biochemistry, Faculty of Science, Jamia Hamdard Deemed University, Hamdard Nagar, New Delhi 110062, India, Tel +91-11-26059688, Fax +91-11-26059663
Received: August 29, 2016 | Published: September 28, 2016
Citation: Routray I, Mahmood A, Ngwa NE, et al. Cell line cross-contamination and accidental co-culture. J Stem Cell Res Ther. 2016;1(5):179-185. DOI: 10.15406/jsrt.2016.01.00031
The cell line cross contamination and co-culture is a major issue in animal cell culture that invalidates the research results, compromises the comparison of results in different laboratories and diminishes the use of animal cell culture for medical purpose and as a viable alternative and an effective tool in understanding the fundamental cell processes. It reduces the quality of the research and may lead to unusable therapeutic products. In stem cell therapy, the engraftment of undifferentiated or incorrectly differentiated cells has been reported to cause substantial tumorigenic or immunogenic risks to the recipient. However, the problem of the undesired or accidental co-culture can be resolved by increasing awareness and following standard procedures, including inspecting regularly the quality of cell lines used in cell culture laboratories. This review provides an insight into accidental co-culture as a result of cross contamination with a brief account of common cross contaminating cell lines and appropriate measures to diminish the chances of cross contamination and accidental co-culture.
Keywords: cell line, cross-contamination, authentication, methods, stem cell, database, cell culture
Contamination of a cell line with another cell in a culture medium, besides infection with microorganisms such as molds, yeast, viruses, protozoa, mycoplasma and bacteria, is a major issue in cell culture experiments. Undesired co-culture or growth of more than one distinct cell types together in a culture medium can be problematic, seriously compromising the quality of results and diminishing the use of cell line as a model system. It may lead to unusable therapeutic product in biotech industry, where a cell line or a manufactured cell product must be fully free from all sorts of contaminations, including the presence of another cell line or its product. Culturing cells together in a combined medium may affect the genotypic and phenotypic stability of the desired cell.1 The genetic instability due to accidental co-culture is a serious issue and this inherent instability in cells like embryonic stem cells and pluripotent stem cells makes it imperative to perform detailed genetic analysis of cells in culture.2 Acceptable degrees of genetic change must be established through chromosomal aberration and karyotyping, cell surface markers and expression of transcription factors, as well as proliferation capacity and differentiation propensity of the cell.3 The accidental co-culture is a particularly serious problem in stem cell therapy, where the engraftment of undifferentiated or imperfectly differentiated cells may cause a substantial tumorigenic or immunogenic risk to the recipient.4,5 This mini review provides a quick insight into the problem of common cross contaminations with undesirable cell line and their co-culture and describes appropriate measures to diminish the chances of contamination of the desired cell line with another cell line and accidental co-culture.
On every occasion, a cell that is maintained in a laboratory faces the risk of contamination with another cell, particularly a rapidly growing and continuous cell line. The first case of cross-contamination in cell culture was reported in 1950s and since then cross contamination (and accidental co-culture) remains a disturbing issue in cell culture laboratories, as contamination of a cell line with another cell line is not readily detectable like the bacterial and fungal contamination.
Among the various cell contaminants, HeLa, the oldest and most commonly used human cell line that was derived from the cervical cancer cells from Henrietta Lacks, taken by George Gey, is perhaps the commonest contaminating cell in cell culture laboratories. The International Cell Line Authentication Committee (ICLAC) database of 475 Cross-Contaminated or Misidentified Cell Lines - originally developed by Amanda Capes-Davis and Ian Freshney (published in 2010) and curated by ICLAC - lists 138 different cell contaminants, with HeLa being the most common, with 113 entries (24%) (http://iclac.org/databases/cross-contaminations/). In an early 1976 study on 246 cell lines over 18 months at The Child Research Center of Michigan, 25% human cell lines were HeLa cells; out of this (246), 30% were reported to be incorrectly designated and 14% were wrong species.6
HeLa has been recognized to be not only the commonest contaminant, but to contaminate a number of cell lines. A PubMed database search for the period between 1969 and 2004 revealed over 60 cell lines that were actually contaminated with HeLa. These HeLa-contaminated cell lines included HEp-2 (laryngeal cancer), KB (oral cancer) and D98/AG. About16 others were contaminated by non-HeLa human cells, while almost 12 cases were that of interspecies contamination.7 According to data published by Capes‐Davis and coworkers,8 and used in this review with permission Figure 1, the maximum number of affected cell lines (106) were found to be contaminated by the human cervical adenocarcinoma (HeLa), followed by human bladder carcinoma (T-24) (18) and human colon carcinoma (HT-29) (12). The other contaminating cell lines in this study included human acute lymphoblastic leukemia (CCRF-CEM) and human chronic myeloid leukemia (K-562), nine each, human lymphoma (U-937) and human acute myeloid leukemia (OCI/AML2), eight each, human esophageal carcinoma (Hcu-10) and human melanoma (M14), seven each, and human acute myeloid leukemia (HL-60), human colon carcinoma (SW-480, SW620) and human prostate carcinoma (PC3), six each. The most frequent contaminants were HeLa, T24 and H29. HeLa has also been reported to be a common contaminating cell in several other studies.9 According to an estimate, HeLa cell contamination is reported to produce financial loss of about 10 million US$.10
The cross-contamination in a cell culture laboratory generally occurs due to failure in adopting good cell culture practices. It may occur at various levels which can be summarized as follows:
Cell line authentication: Methods for the identification of cross contamination
In co-culture, it may not be easy to readily identify different types of cells that grow together unless a specifically designed medium is used to encourage balanced growth Figure 2. In other instances, for example the cross-contamination of normal human fibroblasts (orbital shaped) with HeLa cells (rounded cell colonies), contaminating cell line is easy to identify Figure 3. An important practical approach to learn if a researcher’s own cell line is cross contaminated is to first check previous literature, looking for possible contaminating cells in prior work. In the absence of a prior publication, it is important to check a culture for cross contamination by authenticating testing.9 The Giesma-banded karyotyping and isoenzyme analysis to detect intra- and inter-species contamination of cell lines are two old classical methods to identify contaminating cells.11–14 DNA fingerprinting is a more recent technique that provides valuable input on cell line contamination.15 Cross-contaminated Leukemia cells with three different cell lines (leukemia cell line, acute lymphoblastic leukemia cell line SPI-801, SPI-802, and chronic myeloid leukemia cell line K-562) have been reported to be identified by a multi parameter approach involving cytogenetic examination, DNA fingerprinting and bcr-abl genotyping. The study concluded that the three cell lines were related and had a common origin.14 A list of some authenticated cell line suppliers/authenticating test centers, both stem and non stem cells, and common methods used for authentication is provided Table 1. With regard to stem cell, the NIH Human Embryonic Stem Cell Registry (http://stemcells.nih.gov/Pages/Default.aspx or http://grants.nih.gov/stem_cells/registry/current.htm), beside others, is one of the authenticated sources of stem cells. NIH (http://stemcells.nih.gov) has also formulated guidelines that govern the conduct of stem cell research, particularly the NIH-funded stem cell research.
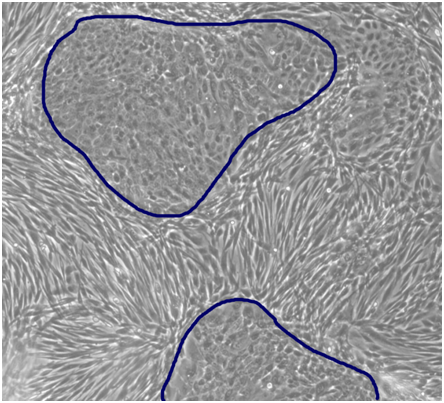
Figure 2 Co-culture of primary human keratinocytes with fibroblasts in CnT-Prime Co-Culture medium specifically designed to encourage balanced growth of keratinocytes and fibroblasts. At confluency, the cultures display the characteristic keratinocyte progenitor cell colonies (small and tight cobblestone morphology) surrounded by fibroblasts (Reproduced with permission from CELLnTEC, http://cellntec.com/products/cnt-pr-cc/#features).
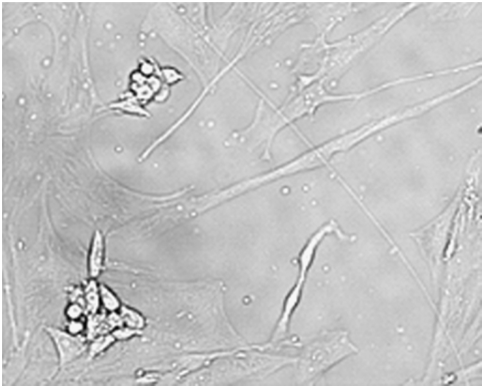
Figure 3 Normal human fibroblasts (orbital shaped) cross-contaminated with HeLa (rounded cell colonies).9
Cell Line Supplier /Authentication Test Centre |
Web Link |
Method used for Authentication |
American Type Culture Collection (ATCC), USA |
www.atcc.org |
STR profiling |
Applied biological materials (Abm), Canada |
https://www.abmgood.com/ |
STR Profiling |
Analytical Biological Services Inc, USA |
http://www.absbio.com/products |
STR profiling |
AllCells, California |
www.allcells.com |
- |
Asterand, Bioscience, USA |
www.asterand.com |
- |
Amsbio, Cambridge |
www.amsbio.com/ |
- |
BioReliance, USA |
http://www.bioreliance.com/in/services/biopharmaceutical-services/ |
CO1 barcode assay, DNA fingerprinting, DNA sequencing, Karyotyping, Random amplification of polymorphic DNA |
Bio-Engineering (I), India |
http://www.tradekeyindia.com/bio-engineering-india/ |
STR profiling |
Biocompare, USA |
www.biocompare.com |
- |
Biosynthesis, Texas |
http://www.biosyn.com/cell-line-services.aspx |
DNA extraction, Unknown sample and known reference standard profiling using STR, Comparison analysis to detect contamination or mutation occurred relatively to the known sample, Bioinformatics data analyses, Electropherogram, Species-specific authentication |
B-Bridge International, USA |
lifesciences.b-bridge.com/ |
- |
Cell Line Genetics®, Wisconsin |
https://www.clgenetics.com/our-services/ |
STR profiling |
Cell Culture and Cytogenetics Facility, University of Pittsburgh |
https://www.scienceexchange.com/labs/ |
DNA Fingerprinting, STR Loci identification by AmpFℓSTR® Identifiler® |
Children’s Medical Research Institute, Australia |
www.cellbankaustralia.com/ |
Human cell line authenticating testing through STR profiling, Non-human cell line authenticating testing through amplification and sequencing of CO1 |
Cell Applications, California |
www.cellapplications.com |
- |
Cell Engineering Division, Japan |
http://cell.brc.riken.jp/en/ |
- |
DDC Medical, Ohio |
http://ddcmedical.com/ |
Human, mouse and canine authentication through STR DNA profiling, Human and mouse pathogen testing, Mycoplasma PCR detection assay |
Dana-Farber Cancer Institute, Massachusetts |
http://moleculardiagnosticscore.dana-farber.org/ |
Human cell line cross contamination identified using DNA fingerprinting with STR |
Duke Clinical & Translational Science Institute, USA |
https://www.ctsi.duke.edu/news/ |
STR profiling to detect misidentified, cross-contamination or genetically drift cell |
DiagCor, Hongkong |
http://www.diagcor.com/en/mdx-consulting-services |
STR profiling |
DSMZ, Germany |
https://www.dsmz.de/home.html |
STR profiling |
DNA Analysis Facility on Science Hill (Yale University), USA |
http://dna-analysis.yale.edu/human-cell-line-authentication-service |
STR profiling |
DNA Sequencing Facility, University of California, USA |
https://mcb.berkeley.edu/barker/dnaseq/node/24 |
Human cell line authentication through STR DNA profiling |
Dkfz. German Cancer Research Center, Germany |
https://www.dkfz.de/gpcf/cell_line_auth0.html |
STR profiling |
EMD Millipore, USA |
www.emdmillipore.com |
- |
Eurofins Genomics, Germany |
https://www.eurofinsgenomics.eu/en/genotyping-gene-expression/ |
STR profiling |
European Collection of Authenticated Cell Cultures (ECACC), UK |
www.hpacultures.org.uk/collections/ |
- |
ESI Bio Stem Cell Solution |
www.esibio.com/ |
- |
Fluidigm, France |
https://www.fluidigm.com/ |
SNP Type Assays |
Gentica Cell Line Testing - a LabCorp brand, USA |
http://www.celllineauthentication.com/services.html |
STR profiling analysis, Mycoplasma contamination detection |
Genetic Resources Core Facility (grcf) - |
http://grcf.med.jhu.edu/ |
STR profiling, Mycoplasma detection |
GenoSeq UCLA Genotyping & Sequencing, California |
http://www.genoseq.ucla.edu/action/view/Other_Services |
GenePrint® 10 system-Promega (STR profiling analysis) |
Garvan Institute of Medical Science, Australia |
http://www.garvan.org.au/research/capabilities/ |
STR profiling, includes comparing results to cellbank and ATCC, Authentication reliability, Matching percentage, Detecting genetic drift, Allelic dropouts, Off-ladder alleles, Checking cross-contamination, Mycoplasma testing |
Global Biological Standards InstituteTM (GBSITM), USA |
https://www.gbsi.org/about/ |
STR profiling |
Human Embryonic Stem Cell Registry, USA |
http://stemcells.nih.gov/ |
- |
IDEXX BioResearch, USA |
http://www.idexxbioresearch.com |
STR profiling, inter- and intra-species contamination check, Comparative analysis of published profile, Genetic profiling using microsatellite markers, Mycoplasma testing |
IdentiCell, Denmark |
http://www.identicell.eu/ |
STR profiling |
Institute for Regenerative Medicine at |
https://medicine.tamhsc.edu/irm/msc-distribution.html |
- |
InvivoGen, California |
www.invivogen.com |
- |
Japanese Collection of Research Bioresources (JCRB) Cell Bank |
http://cellbank.nibiohn.go.jp/english/cellinfo_e/ |
- |
Korean Cell Line Bank, Korea |
cellbank.snu.ac.kr |
STR profiling |
Laragen Sequencing & Genotyping, California |
http://www.laragen.com/laragen_cellline.html |
STR profiling |
Leibniz-Institut DSMZ - Deutsche Sammlung von |
www.dsmz.de/ |
- |
Lonza, India |
www.lonza.com |
- |
Microsynth The Swiss DNA Company, Switzerland |
http://www.microsynth.ch/index.php?TPL=10581 |
STR profiling, |
Mycoplasma contamination testing of cell culture supernatant |
||
MHTP Medical Genomics Facility, Australia |
http://www.mhtpmedicalgenomics.org.au/index.php/ |
STR profiling |
Multiplexion, Germany |
http://www.multiplexion.de/en/cell-line-testing-service/ |
Identification of human cell lines by SNP profiling, STR profiling |
Miltenyi Biotec, California |
www.miltenyibiotec.com |
- |
Mirus, USA |
www.mirusbio.com |
- |
MTI-GlobalStem, USA |
www.mti-globalstem.com |
STR profiling |
NIH AIDS Reagent Program, USA |
https://www.aidsreagent.org/index.cfm |
- |
Northgene, UK |
http://www.northgene.co.uk/ |
DNA STR analysis |
Provitro, Germany |
http://www.provitro.com/ |
- |
Public Health England (PHE), UK |
https://www.phe-culturecollections.org.uk/ |
STR profiling |
Promocell, India |
www.promocell.com |
- |
Promega, Sweden |
http://www.promega.in/ |
STR profiling |
RIKEN Bioresource Center Cell Bank, Japan |
www.brc.riken.go.jp/lab/cell/english/ |
STR profiling |
Reachbio Research Lab, USA |
reachbio.com/ |
- |
ScienCell Research Laboratories, California |
www.sciencellonline.com |
- |
Sigma-Aldrich, USA |
www.sigmaaldrich.com |
- |
Stemgent, Massachusetts |
www.stemgent.com |
- |
The Translational Research Initiatives |
https://www.pathology.wisc.edu/research/trip |
STR profiling |
Thermo Fisher Scientific, USA |
www.thermofisher.com |
- |
The National Centre for Cell Science (NCCS), India |
www.nccs.res.in/ |
- |
University of Arizona Genetics Core, Arizona |
http://uagc.arl.arizona.edu/services/ |
STR profiling |
University of Florida Interdisciplinary Center for |
http://www.biotech.ufl.edu/now-offering-human-cell-line |
STR profiling analysis performed using GenePrint 10 STR system |
Uppsala Universitet, Sweden |
http://www.igp.uu.se/ |
STR profiling |
WiCell, USA |
http://www.wicell.org/home/stem-cell-lines/ |
- |
Table 1 List of some cell line suppliers/authentication test centres, both stem cell and non stem cell, and common methods used for authentication
Precautions to check cross-contamination and accidental co-culture
Utmost care is required while handling cell lines, especially when managing several cell lines together. The precautionary measures include handling one cell line at a time, proper labeling of each culture flask with name of the cell line, passage number and transfer date. The media for each cell line should be kept in separate tubes. Serological pipettes must be discarded after each operation. Used media and fluids must be discarded in separate containers. The hands and workbench must be cleaned with 70% alcohol before and after each operation, particularly while starting with the second cell line. Bottles, aliquots of the medium and other reagents should be dedicated for single cell line. These regents and aliquots of the medium should be labelled properly and never be shared between cell lines. The used waste pots should be replaced with disinfected ones. The cell line should be purchased from reputed cell bank and a periodic check of its morphology and growth characteristic should be conducted in phase contrast microscope. Good aseptic technique and lab practices should be practiced and techniques such as the short tandem repeat profiling, DNA fingerprinting, karyotype analysis and isoenzyme analysis through electrophoresis should be employed to periodically check cross contamination and reduce the risk of accidental co-culture. The cross contamination, if detected, should be reported to cell repository data bank to avoid use of suspected cell lines.15
Cell line cross-contamination and co-culture is a serious problem in cell culture laboratories as it is difficult to identify when compared with microbial contamination. The contamination of a cell line with another cell line and accidental co-culture invalidates the research results and has serious implications in terms of therapeutic and industrial use of cell lines or their products. In this review, we attempted to provide a quick insight with regard to cross contamination with common contaminating cells, reasons for cross-contamination, most common contaminating cell lines and methods to detect contamination and check accidental co-culture.
Authors acknowledge the UGC SAP grant and DBT supported Bioinformatics Infrastructure Facility at JH. NEG acknowledges ICGEB SMART Fellowship.
The author declares no conflict of interest.

©2016 Routray, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.