Journal of
eISSN: 2475-5540


Review Article Volume 1 Issue 4
Department of Biology, Islamic Azad University, Iran
Correspondence: assan Dana, Damghan Branch, Islamic Azad University, Damghan, Iran, Tel 0098 91050 40602
Received: July 16, 2016 | Published: September 20, 2016
Citation: Dana H, Mahmoodi G, Marmari V, et al. An overview of cancer stem cell. J Stem Cell Res Ther. 2016;1(4):169-174. DOI: 10.15406/jsrt.2016.01.00029
Stem cells are determined as cells with the capability to perpetuate themselves through self- renewal and to produce mature cells of a special tissue through differentiation. Due to the tremendous clinical and biological significance of cancer stem cells (CSCs), research related to these cells is rapidly evolving. Although a series of hypotheses have been proposed about the origin of CSCs, their origin is yet to be discovered. CSCs play a critical role in cancer creation, evolution, metastasis, and recurrence. The existence of CSCs is deeply applied in cancer therapy, since they have a collection of markers for detection and determination. This article reviews the characteristics of CSCs in terms of their origin, their application in CSCs and cancer therapy, and their isolation techniques.
Keywords: cancer, the cscs theory, stem cell
CSC, cancer stem cell; TIC, tumour initiating cell; EMT, epitheliale mesenchymal transition; FACS, fluorescence activated cell sorting; MACS, magnetic cell sorting
Cancer is described as malignant cells that display excessive and uncontrolled expansion of abnormal cells with functional heterogeneity and proliferative. In tumor cells, the changes in shape, size, nucleus/cytoplasm ratio, thickness, etc, are first studied and morphological heterogeneity is further manifested.1–3
Given that at various stages of maturation, tumors contain heterogeneous populations of cells and also the properties of normal stem cells shared by cancer cells led to the speculation that tumors may contain a cancer stem cell population that drives the growth of the tumor.4
Cancer stem cells (CSCs) defined as a subpopulation of tumor cells that possess stem cell characteristics of differentiation into various cell types and unchecked self-renewal and high tumorigenic activity.5,6 In cancer patients, high rates of therapeutic failure seen correlates to accumulation of drug-resistant CSCs.6
Various methods have been employed to purify, identify, characterize, and enrich CSCs in different tumor systems. For the first time, in 1994 from acute myelogenous leukemia and later in solid tumors of various organs, such as the brain, colon, liver and lung, CSCs were discovered.6,7
Though CSCs have properties similar to normal stem cells, but, it is not obvious whether CSC arise through the transformation from conversion of cancer cells to undifferentiated stem-like cells or multipotent adult stem cells. CSC represents an important target in cancer treatment for killing cancer cells or preventing cancer recurrence because these cells have been recognized as tumor-initiating cells (TICs) or tumorigenic cells.8
The concept of the stem cell hypothesis of cancer notices that only a small subset of long-lived cells with dual potential can proliferate unlimitedly, which gives rise to macroscopic metastases, and reinitiates tumor formation upon serial transplantation of the low cell number into immunocompromised mice. The dual potential of stem cells was both interesting and unknowns for decades, because the usual understanding was that any dividing cell gives rise to two identical daughter cells with equal distribution in all cellular compartments. Though, it is now obvious that natural stem cells and cancer stem cells have an inherent ability to divide asymmetrically,9 generate two cells with difference in chromatin and DNA, centrosome composition, size, developmental potential, inheritance, protein content etc, the molecular mechanisms of these processes have yet been completely unclear.10 It was shown that multiple important regulators of the asymmetric cell division act as tumor suppressors.11,12
According to the CSC hypothesis, similar to normal tissues, cancers are hierarchically organized and cancer growth and progression are driven by a small subpopulation of tumor cells with stem cell-like properties, the CSCs13 (Figure 1).
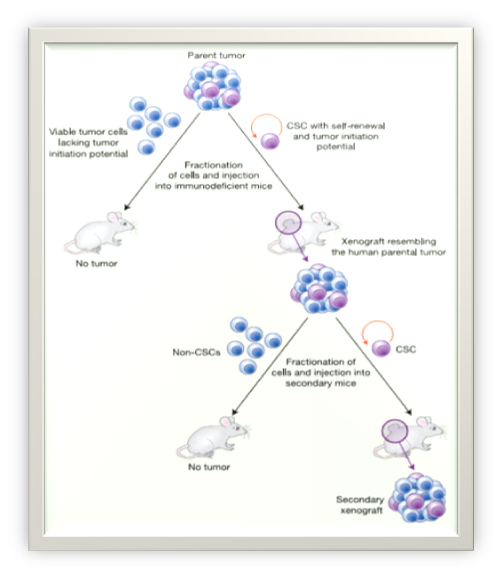
The CSCs theory
Cancer stem cell (CSC) concept is one the hypothesis proposed to explain the tumor cell heterogeneity. This hypothesis postulates that, like to growth of normal proliferative tissues, growth of tumors or development of a tumor clone is driven by a population of cells endowed with both differentiation abilities and self-renewal; however, self-renewal is typically deregulated in CSCs. CSCs, as with normal stem cells, are longlasting and have self-renewal abilities. Also, proliferative potential with stem cells as the common denominator in regenerating normal tissues and human cancers are organized in a hierarchical manner according to stages of differentiation. Although, this does not necessarily mean that CSCs are always derived from normal stem cells. A CSC is set apart from a normal stem cell in that it has acquired the capacity for indefinite proliferation through accumulated genetic mutations and epigenetic alterations. In this case, tumorigenesis occurs, when signaling pathways that regulate normal stem cell self-renewal are dysregulated.14
Functional analytical techniques, modern genomics and proteomics, have considerably deepened our knowledge in cancer molecular biology.15 Studies on tumor heterogeneity and relevant mechanisms have verified the presence of a unique portion of tumor cells possessing unique self-renewal, proliferation and differentiation ability, called tumor initiating cells (TICs) or cancer stem (-like) cells (CSCs).16,17 A number of several intracellular dyes and particular cell surface markers are now utilized to isolate or detect CSCs from solid tumors and haematopoietic malignancies. CSCs are believed to obtain differentiation and self-renewal capabilities, and to give rise to more differentiated derivatives, which greatly comprise the bulk of tumor tissues.18,19 Several experiments have also proposed an important role of epithelial mesenchymal transition (EMT) programs in generating cells with the traits of CSCs in various cancers.20
Origin of cancer stem cells
The CSC supposition about the origin of cancer is an updated copy of “embryonal rest theory” proposed in more than 150 years ago that accounts the likeness between a definite tumor, like teratocarcinomas, and a developing embryo, which has enormous differentiation and proliferative capacity.21,22 The defeat of common therapies to eradicate cancer by a definitive method can be easily percepted by examining the side effects of current cancer treatments and studying how they vanish once the treatment is stopped.23
The origin of CSCs within solid tumors remains elusive; some studies indicate that they may originate from a series of naturally-occurring normal stem cells or from differentiated cells.24 Although cancer-initiating cell and cancer stem cell are sometimes used interchangeably, the initial cell that develops cancer is not necessarily a cancer stem cell. The presence of CSCs was first introduced 40years ago, although analysis of its details remains a secret until developing advanced research tools.25 The best proof to verify the presence of CSCs came from the research of hematological malignancies.26 Due to the function of embryonic stem cells and self-renewal in adult cells, like blood cells, the description of CSCs was revealed27 (Figure 2).
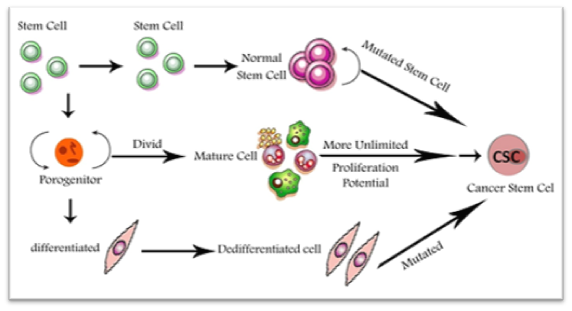
Isolation of CSCs and markers
FACS (Fluorescence-activated cell sorting), long term cell culture, and MACS (magnetic cell sorting) are the basic techniques used to isolate Cancer Stem Cells. CSCs enrichment can be accomplished using the FACS technique, which is according to the expression of specific proteins in cell culture, epigenetic changes, expression pattern and cellular-level of markers such as CD44, CD133, CD 24 and ALDH1. CSC characteristics can be defined via copy number variation, mRNA and miRNA expression analysis, and etc.
MACS (Magnetic Cell Sorting): This technique can isolate cells based on the expression of specific stem cell markers like CD166. In this method, prior to isolating, cell markers are labeled using a specific magnetic Microbead like anti-or a monoclonal antibody CD166 which is 106 times smaller than the cell’s size. Followed by labeling and magnetic isolation, washing is performed. Then the marked cells are isolated by positive selection.28
CSCs have a collection of markers for detection and determination. For example, CD166 known as an activated leukocyte cell adhesion molecule is an immunoglobulin protein and a special surface antigen in lymphoid stem cells. Though, the function of CD166 is still to be discovered, it is recognized as a marker for cancer tissues and is used individually or combined with other markers to isolate stem cells from many tumors like breast, colorectal, prostate, and etc (Table 1).29
Cell Surface Marker |
Tumor Type and References |
CD133 |
|
CD34 |
Acute myeloid leukemia57 |
CD44 |
Colon,58 Prostate,59 Breast,60 |
CD45 |
Hepatocellular71 |
CD49 |
Prostate12 |
CD15 |
|
CD166 |
Breast,65 Colorectal,51 Prostate, Melanoma, Bladder, Esophageal Squamous Cell Carcinoma52 |
CD326 |
Bladder, Breast, Prostate, Colon, Lung66 |
CD117 |
Lung12 |
CD90 |
Lung12 |
CD24 |
Pancreas,58 Ovarian67 |
CD26 |
Colon28 |
CD38 |
Acute myeloid leukemia57 |
CD20 |
Melonoma12 |
CD29 |
Breast60 |
CD271 |
Melonoma12 |
ALDH1 |
Breast,68 Gastric,69 Lung70 |
Table 1 Surface markers and phenotypes of different CSC
Targeting CSCs
Radiotherapy, surgical resection and chemotherapy: These are three main strategies for conventional cancer therapy. Targeting CSCs is a new prospect for cancer therapy created by cancer stem cell theory, therefore, an important part of cancer therapy should be CSCtargeted therapy.30
The existence of CSCs has deep uses for cancer therapy.5 Whereas, CSCs are more resistant to conventional therapies, compared to differentiated cells constituting the tumor bulk, therefore the combination of drugs directed against CSCs and conventional chemotherapy would have the potential to overcome tumor resistance that eventually improves patient survival and reduces relapse.31 It was proposed that targeting CSCs could be gained by some strategies such as inducing the differentiation, inhibiting the self-renewal signaling and sensitizing them to chemotherapeutic agents.32–34
CSCs are largely impressed by signals from microenvironment and moreover rely on signal transduction pathways nearly related with stem-cell biology, both of which are druggable targets.35,36 Detecting the place of CSCs and monitoring their physiologic indexes in timely is hard. So, expanding techniques that can be applied to study CSCs will have a significant impact on therapy and clinical diagnosis for cancer.
Capability to target CSC therapeutically dependents on identifying the underlying survival signaling pathways and their unique cell surface markers. For example, pathways mediated by Akt, Wnt, PTEN, BMP, FGF, notch, and hedgehog are several key signaling pathways which have been linked with maintenance and the self-renewal of CSC.37 Targeting specific CSC-relevant signal transduction pathways seems reasonable and promising; but; actually it indicates a complex issue due to redundancy of the participating elements and the extreme diversity.38
In most cancers, distant metastasis, tumor aggressiveness, histologic grade and poor prognosis correlate with the ratio of CSCs.39–47 For instance, in metastatic colorectal cancer for standard first-line bevacizumab-based treatment, CD166 was shown to be a predictive marker48–51 or in patients with gastric cancer, CD133- and CD44- positive had a poorer survival rate than patients with CD133- and CD44- negative.52
Several natural extracts are claimed to target CSCs. It was reported that γ-tocotrienol can reduce the expression level of CSC surface markers on prostate tumor cells. A new door for CSC targeting in cancer therapy research may be open by this finding and usage of natural extracts targeting CSCs.
In spite of all the studies and analyses on CSCs, all strategies for treatment are under-test theories. Due to the tremendous clinical and biological significance of CSCs, research related to these cells is rapidly evolving. CSCs have had a deep impact on our current view of cancer treatment, prognosis, and diagnosis. According to experimental evidences indicated compared to monotherapies, treatment strategies based on the combination of conventional therapies targeting CSC specific pathway inhibition and bulk tumor cells bear a promise to improve cancer cure rates. CSC hypothesis does provide a new prospect in cancer therapy. It is believed that there are a small number of stem cells at the center of tumor sustaining malignant tissue growth. Efficiency and success of the cancer treatment in the first stage is measured by tumor mass cutting, however, cancer stem cells can yet form very small part of tumors through their activity and form new tumors. It should be considered that these cells induce only tumor mass and are not able to produce new cells and do not have role in disease progression and tumor growth, while the populations of cancerous cells cause cancer, tumor growth and also recurrence of the disease, out of sight and remain intact. If it is true, then it could be explained why tumors are often restored even after they have been almost destroyed by anticancer drugs (Figure 3). This also points to a different strategy to develop anti-cancer drugs, suggesting that these drugs must be chosen to destroy cancer stem cells and not for their ability to kill just any cells or shrink tumors.
None.
The author declares no conflict of interest.

©2016 Dana, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.