Journal of
eISSN: 2475-5540


Mini Review Volume 1 Issue 2
School of Biomedical Sciences, The University of Hong Kong, China
Correspondence: Martin Cheung, School of Biomedical Sciences, Li Ka Shing Faculty of Medicine, The University of Hong Kong, Hong Kong, Tel 852 3917 6867
Received: May 13, 2016 | Published: June 2, 2016
Citation: Liu JS, Cheung M. Neural crest stem cells/progenitors and their potential applications in disease therapies. J Stem Cell Res Ther. 2016;1(2):80-87. DOI: 10.15406/jsrt.2016.01.00014
The neural crest is a remarkable embryonic population generated transiently during early vertebrate development. Because of their multipotency and extensive migratory capacity, neural crest progenitors harbor stem cell characteristics with self-renewal capacity and contribute to a variety of differentiated cell types from cranio-facial skeletal tissues to peripheral nervous system in the trunk. Multiple molecules including signaling factors, transcription factors and components of migratory machinery are expressed at different stages of neural crest development. Gain- and loss-of -function studies in several vertebrate species have revealed the functional relationship of these molecules and assembled them into a gene regulatory network that define the process of neural crest induction, specification, migration and differentiation. These studies form the fundamental criteria for the subsequent establishment and molecular validation of neural crest stem cells/progenitors derived by various strategies. We present here in vivo and in vitro characterization of neural crest stem cells isolated from embryonic, fetal and adult tissues as well as the latest experimental approaches for their derivation from embryonic stem cells, induced pluripotent stem cells and skin fibroblasts. We further provide an overview of the recent development in applying neural crest stem/progenitor cells for the treatment of neural crest-associated diseases. Future work is required to explore the possibility in directing neural crest -derived specific lineages from fibroblasts using transcription factor-mediated reprogramming strategy, characterize the differentiation potential of adult-derived neural crest stem cell from different tissue origin, and use genomic editing approach to correct genetic mutations in patient-derived NCSCs for transplantation therapy. These endeavors should further unravel and enhance the therapeutic potential of neural crest stem cells/progenitors in clinical setting.
Keywords: neural crest stem cells, neurocristopathy, induced pluripotent stem cells, human embryonic stem cells
NCSCs, neural crest stem cells; EMT, epithelial-mesenchymal transition; PNS, peripheral nervous system; N-Cad, n-cadherin; Cad7, cadherin7; DRG, dorsal root ganglia; SKPs, skin precursor cells; FD, familial dysautonomia; ESCs, embryonic stem cells; iPSCs, induced pluripotent stem cells; m, mouse; h, human; INC, induced neural crest; HSCR, hirschsprung; S-HSCR, short-segment hirschsprung; L- HSCR, long-segment hirschsprung; RA, retinoic acid
The Neural crest stem cells (NCSCs) represent a transient stem-cell like population that emerges from the dorsal neural plate border during gastrulation. Inductive signaling molecules derived from the non- neural ectoderm and underlying mesoderm contribute to the specification of the border region where multipotent NC stem cells (NCSCs)/progenitors are formed. They then undergo morphological change in a process called epithelial -mesenchymal transition by which neuro epithelial NCSCs/progenitors detach from its neighboring cells through alteration of cell-cell adhesion and cytoarchitectural properties, resulting in acquisition of mesenchymal migration as they delaminate from the dorsal neuroepithelium (or premigratory NC domain). Depending on axial origin and environmental guidance cues, NCSCs/progenitors migrate along stereotypical routes to colonize various embryonic regions, where they differentiate into an astonishing array of cell types and tissues, such as the ectomesenchyme of the craniofacial elements (cartilage and bones), sensory neurons and enteric ganglia of the peripheral nervous system (PNS), melanocytes in the skin, as well as smooth muscle cells of the cardiac outflow tract.
A cascade of molecules involved in different steps of NC development are functionally linked and integrated in a gene regulatory network that define their developmental features at different stages. Because of their multipotentiality, genetic mutations result in dysregulation of NC development and many congenital human diseases including craniofacial abnormities, cardiovascular defects, and intestinal aganglionosis, collectively known as neurocristopathies.1,2 In addition, the ability to identify and isolate multipotent NCSCs during embryogenesis and in the adult tissues as well as their derivation from embryonic stem cells (ESCs), induced pluripotent stem cells (iPSCs) and skin fibroblasts are promising cellular sources for the treatment of various neurocristopathies. In this review, we provide an overview on key regulators in NC development, the strategies for the isolation and characterization of NCSCs/progenitors, and the specific conditions required to differentiate stem cells or reprogram somatic cells into NCCs, as well as their therapeutic potential for the treatment of neurocristopathies.
Molecular regulation of neural crest induction and EMT
NC induction process spans the period from early gastrula stage to neural tube closure. Tissue grafting experiments in amphibian and chick embryos have established that interactions between the neural plate and the non -neural ectoderm result in NC formation.3,4 In addition, interaction between paraxial mesoderm and neural plate is also sufficient to induce NCSCs in vitro.4,5 Gain and loss of function studies in the mouse, chick, frog and zebrafish have demonstrated the involvement of BMP, Wnt, Notch/Delta and FGF signaling molecules derived from these tissues for induction of NC formation within the neural plate border region.6–19 Although their relative importance and the timing of their action varies between species, it is believed that combinatorial activity of these signaling molecules are crucial for the establishment of neural plate border region distinct from the neural and non-neural ectoderm. In response to the early inductive signals, neural plate border cells turn on a distinct set of transcription factors called neural plate border specifiers including Tfap2, Msx1, Zic1, Pax3 and Pax7.20–26 These factors once initiated maintain their expression by forming regulatory interactions between each other,27,28 and together with neural crest signaling pathways to specify NC progenitors within the neural plate border by activating the expression of NC specifiers including those encoding transcription factors of Snail, FoxD3 and SoxE family (mainly Sox9 and Sox10).29–32 Numerous functional studies in several species have revealed the importance of these molecules in defining NC identity.32–37 In most cases, over expression and knockdown of one of the NC specifiers affect the expression of another one, indicating that like neural plate border specifiers, genes involved in specification form inter- connected regulatory loop that is believed to be crucial for maintaining NC progenitors in a multipotent state.38–41 In addition, the NC specifiers also serve additional function by conferring NC progenitors with ability to undergo an EMT through alteration of cell-cell adhesion properties and regulation of Rho GTPases, which are essential for remodeling of actin cytoskeleton dynamics.39,42,43 Previous studies showed that FoxD3 regulates the switching from N-cadherin (N -Cad) to cadherin 7 (Cad7) expression. Sox9 cooperates with Snail2 to specify NC identity and promote an EMT by inducing morphological change and migratory behavior of NCSCs.39,44 Therefore, combined expressions of NC specifier genes manifest the characteristic features of delaminating NCSCs. How Sox9 and Snail2 exert their influence on NC motility remain to be elucidated. Together, these studies have established a hierarchical gene regulatory network to orchestrate NC formation (Figure 1).
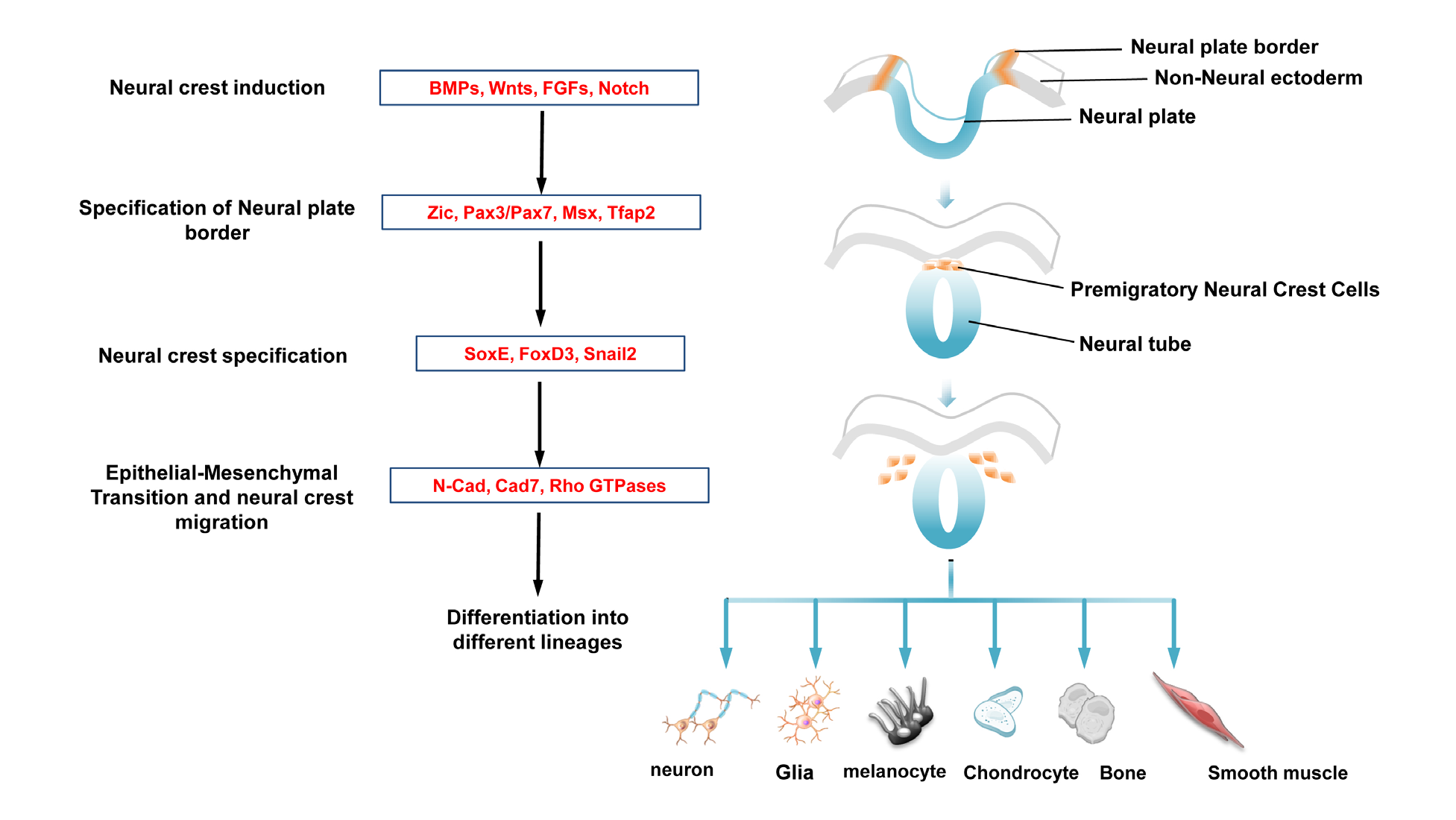
Figure 1 A gene regulatory network regulates neural crest (NC) development.
Diagram shows representative molecules in each regulatory module are functionally linked to form a gene regulatory network. Inductive signaling molecules secreted from the surrounding tissues of the neural plate activate expression of neural plate border specifiers in the dorsal neural folds, where subsequent initiation of NC specifiers expression define cells with NC identity. It is conceivable that the transcriptional outcome of NC specifiers bestow NCSCs with ability to undergo epithelial-mesenchymal transition through alteration of cell-cell adhesion properties and regulation of Rho GTPases for cyto skeletal remodeling. After delamination from the dorsal neural tube, NCSCs migrate to their target destinations, where they differentiate into a variety of functional cell types.
Neural crest derivatives and their signaling regulation
After delamination, NCSCs undergo extensive migration following stereotypical routes and eventually settle and differentiate into different derivatives,1,45 which are mainly determined by the axial origin of the cell within the neural tube, as well as by complex sets of environmental cues they encounter during migration. Cranial NCSCs derived from the mid-diencephalon to somite 5 contributes to the craniofacial structure of the head by forming majority of the cartilage and bone tissues of the skull, facial and pharyngeal skeletons as well as cranial neurons, glia, and connective tissues of the face. The trunk NCSCs, which arise from the neural tube caudal to the somite 5, follow two distinct migratory pathways. The first migratory route takes the trunk NCCs ventrolaterally through the anterior portion of sclerotome where they form the dorsal root ganglia (DRG) containing the sensory neurons and satellite glial. Those continue to migrate ventrally form the Schwann cells along the spinal nerves, sympathetic ganglia and endocrine cells of the adrenal gland. The second migratory pathway occurs dorsolaterally beneath the ectoderm to form pigment-producing melanocytes. A subpopulation of trunk NCSCs lying opposite somites 1-7 (vagal) and posterior to somite 28 (sacral) contributes to the formation of the enteric ganglia throughout the entire gut. Finally, the cardiac NCSCs derived from the neural tube lying adjacent to somites 1-3 which overlaps with the anterior portion of the vagal region, give rise to the muscle and connective tissue wall of the large arteries as well as the septum of the outflow track.
It has been well established that similar set of signaling molecules involved in NC induction also play important roles in NC differentiation. For example, Wnt signaling has been implicated in specifying trunk sensory neuron formation. Activation and inhibition of canonical Wnt signaling in the NC progenitors result in promoting and lack of sensory neuron formation respectively.46,47 In addition, BMP2 and BMP4 secreted by the dorsal aorta induce the differentiation of migrating NCSCs into sympathetic neuron.48–50 FGF signaling plays a crucial role in determining skeletogenic fate of cranial NCSCs. Functional studies in mice and chick demonstrate that cranial NCCs expressing Fgf receptor 1 enter the branchial arches where they respond to the FGF ligands secreted by the pharyngeal ectoderm and differentiate into cartilage tissue.51–54 Finally, Delta/Notch signaling mediated lateral inhibition to determine the neuron-glial fate of NC progenitors in the DRG.55
Neural crest stem cells
Studies using in vitro clonogenic assays, in vivo transplantation, and cell labeling revealed that premigratory NCSCs are a heterogeneous population of multipotent and self-renewing NC progenitors.56–60 In contrast, other studies proposed that the NC was comprised of heterogeneous population of fate-determined progenitor cells and the type of derivatives generated depend on their spatial-temporal distribution in the premigratory region.61,62 Recent in vivo fate mapping studies provide definite evidence that most of the premigratory and postmigratory murine NC populations are multipotent.63 The idea of NC stem cells (NCSCs) was originally proposed by Stemple & Anderson as demonstrated by their ability to isolate a pure rodent NC population expressing p75NTR with multipotency and self-renewal capacity in vitro.64 Subsequently, the same research group isolated NCSCs from post-migratory NCC populations in rat fetal sciatic nerve that gave rise to neurons, Schwann cells, and smooth muscle-like myofibroblasts in vitro.65 In addition, an undifferentiated progenitor population derived from early migratory cranial NCSCs in mouse and chick embryos exhibit self-renewal capacity and differentiation into cell types as diverse as neurons, melanocytes, osteocytes, and chondrocytes.66–68
The existence of multipotent NCSCs is found not only in the early embryonic stage but also in the fetal period and adulthood from different vertebrate species. For example, multipotent NCC populations derived from the cardiac side population in the neonatal and adult mouse heart are able to form cardiospheres. Upon transplantation into chick embryos, cells derived from cardiospheres are able to migrate and differentiate into trunk NC-derived PNS neurons and peripheral nerve.69 In addition, enteric progenitors derived from the fetal gut exhibit a greater extent of self-renewal and differentiation capacity than adult gut progenitor cells.70,71 Another key finding was the identification and isolation of multipotent NC progenitors from adult rodent facial hair follicle and dermal papilla.72,73 These skin precursor cells (SKPs) were able to proliferate and differentiate into neurons, smooth muscle cells, Schwann cells, and melanocytes in vitro. Furthermore, transplantation of SKP-derived neurospheres into chick embryos showed their migratory capacity and colonization to NC- derived structures, such as the DRG and the peripheral nerve.72
In addition to isolation of NCSCs/progenitors from the embryonic and adult tissues in animal models, recent efforts have been focusing on their derivation from human (h) embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) to understand their biology and pathology of neurocristopathies as the availability of early gastrula human embryos for experimental manipulation is very limited. Nevertheless, expression of NC genes in different stages of human embryos and in human NCC lines derived from pharyngulas has been documented.74,75 These studies revealed several NC genes are commonly expressed between human and other animal models such as neural plate border specifiers (Pax7, Pax3, Msx1) and NC progenitors (Sox9, Sox10, Snail2, Foxd3) and they have been used as markers to identify both mouse (m) and hESCs/iPSCs-derived NCSCs.76 However, transcriptome analysis further revealed that markers for ESCs, such as OCT4, NANOG and SOX2 are also expressed by hNCSCs but not NCSCs/progenitors derived from other animal species, indicating that hNCSCs exhibit unique molecular and phenotypic features of stem cells.75 In addition, HNK1 (carbohydrate) epitope) has been widely used to mark migrating NCSCs in chick embryos77 but it only labels a small population of hNCSCs. In contrast, p75 (nerve growth factor receptor) marks a large amount of hNCSCs and also other non- NC cell types.74 Therefore, FACS using antibodies against both surface markers could further enrich hNCSCs differentiated from a mix population of cells.
Mizuseki & colleagues first established culture conditions in the presence of PA6 stromal cells that induced mouse and primate ESCs to differentiate into NCCs when BMP4 was added after 4 days of co-culture. These ESCs - derived NCCs could be further differentiated into sensory and autonomic lineages by low and high levels of BMP4, respectively.78 Subsequent studies using stromal cells feeders layer also successfully differentiated hESCs into hNCSCs and their PNS derivatives such as sensory and sympathetic neurons.79–81 Further improvement of the protocol without a feeder layer revealed the ability to differentiate into NCSCs/progenitors from human (h)ESCs and hiPSCs via the neural rosette stage in a more efficient manner than using feeders layer.82,83 Upon differentiation of hESCs/hiPSCs into neural rosettes at high density in droplets in the absence of feeder, NCCs emerge at the rosette border and are further enriched and isolated by FACS using HNK -1/p75 antibodies after culture in medium containing inhibitors blocking TGF-β and BMP signaling.83 The enriched NC precursor population plated at clonal density can be passaged and differentiated into peripheral neurons, Schwann cells, osteogenic, adipogenic, chondrogenic cells, and smooth muscle. In vivo transplantation assay further demonstrated their survival, migration and differentiation compatible with NC identity.82
Since it is impossible to isolate human fetal NCSCs due to ethical and experimental challenges, patient-specific iPSCs-derived NCSCs provide a new platform for modeling human neurocristopathies to understand pathogenesis and also for validating candidate drugs. More importantly, since NCSCs are derived from the patients, they can be used for autologous transplantation without immuno-rejection. Following the establishment of an efficient protocol for the differentiation of human iPSCs into neuroectodermal and NCSCs based on pharmacological inhibition of dual Smad pathways,84,85 a major advance was achieved by using a single transcription factor, Sox10, to generate multipotent induced NC (iNC) cells from human postnatal fibroblasts (Figure 2).86 Sox10 broadly marks all the NCSCs during development, and is essential for the maintenance of their multipotency, self- renew, survival, and lineage-specification.87 Therefore, lentiviral-mediated over expression of Sox10 together with extracellular matrix components and epigenetic modifiers was able to direct reprogramming of human fibroblasts into iNC cells. Importantly, addition of the Wnt agonist, Chir99021, into the culture medium further increase the number of iNCs, which exhibited similar morphological and cellular features as well as gene expression profiles to hESC-derived NCSCs. Further in vivo and ex vivo transplantation assays revealed that these iNCs were able to migrate and integrate into NC- derivatives, such as the dorsal root and sympathetic ganglia and the enteric nervous system.86 It should be noted that one of the major concerns for iNCs generation is the lentiviral integration into the host genome that may result in tumor formation and is not ideal for therapeutic applications. Nevertheless, the lineage conversion strategy to generate multipotent iNCs provides an accessible platform for studying human NC biology and the pathogenesis of neurocristopathies.
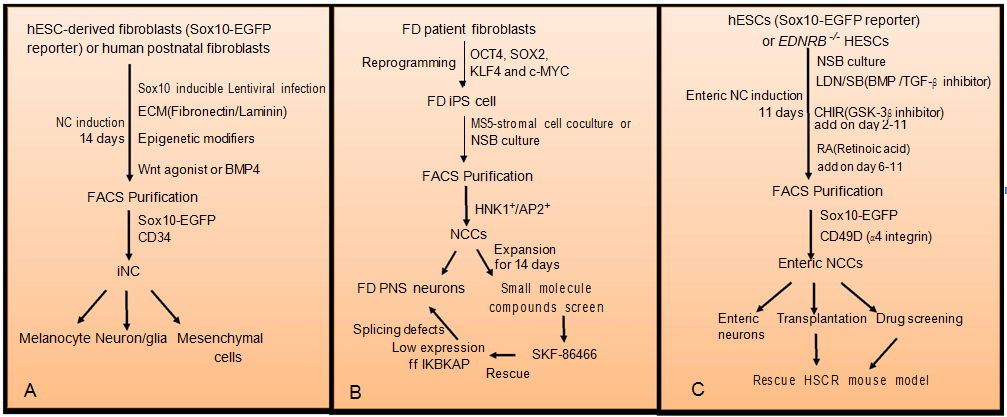
Figure 2 Strategies for human neural crest cells (hNCSCs) derivation and their applications in disease modeling.
(A) Human embryonic stem cells (ESCs)- derived fibroblasts harboring SOX10-EGFP reporter or postnatal is directly reprogrammed into NCCs by lentiviral- mediated over expression of SOX10 together with a combination of extracellular matrix (ECM) components (fibronectin/lamin), epigenetic modifiers, and Wnt agonist or BMP4. Induced NC (iNC) cells expressing SOX10-EGFP or CD34 are further enriched by flow cytometry. Purified iNC can differentiate into different derivatives. (B) Familial dysautonomia (FD) patient fibroblasts are reprogrammed into induced pluripotent stem cells (iPSCs) after transduction with four Yamanaka factors (OCT4, SOX2, KLF4 and c-MYC). The FD-iPSCs are further differentiated into NCSCs by using MS5 stromal coculture or NSB culture. HNK1+/AP2+ FD- NCSCs are purified by flow cytometry and expanded for 14 days. FD-NCCs are then subject to PNS neuronal differentiation for disease mechanism study or small molecules screen for therapeutic development. (C) hESC (Sox10 -EGFP reporter) or EDNRB -/-hESC are induced to enteric NCSCs by using a dual SMAD inhibition (DSi) protocol with optimized level of BMP, TGF-β, Wnt and RA signaling. Enteric NCSCs expressing CD49D and SOX10-EGFP are enriched by flow cytometry. Purified enteric NCSCs are subject to enteric neuronal differentiation, in vivo transplantation and drug screen for HSCR therapeutic treatment.Neural crest-associated diseases and stem cell therapy
Defective migration, proliferation and differentiation of NCSCs are associated with a number of congenital diseases due to genetic mutations, and many of which primarily affect pediatric patients. In addition to human disease modeling, patient-specific iPSCs-derived NCCs or iNCs are viable cellular source for transplantation therapy and drug screening.
One of the neurodegenerative diseases in PNS is Familial dysautonomia (FD), which is an autosomal recessive disorder characterized by autonomic dysfunction including progressive loss of sympathetic and sensory neurons, resulting in gradually diminished pain and temperature sensation. This is mainly caused by a single point mutation in the ELP1/IKBKAP (a subunit of Elongator complex) gene in the germline, resulting in IFBKAP mis-splicing and a marked reduction of IKBKAP protein expression.88 In mice, conditional knockout of Ikbkap in NCSCs caused aberrant neuronal differentiation and early neuronal death.89 To investigate the underlying causes of FD, Lee and colleagues generated iNCs from FD skin fibroblasts and showed that FD-derived iNCs exhibited migration defect compared to the control-iNCs. Consistently, gene expression profiling studies further revealed that down regulated genes in FD-iNCs were involved in alternative splicing and cell migration.86 More importantly, one of the small molecules, SKF -86466, was identified to restore both IKBKAP protein and autonomic neuronal marker expression lost in FD90 (Figure 2).
In addition, another neurocristopathy being well studied is Hirschsprung’s disease (HSCR), also called aganglionic megacolon, which affects 1:5000 new borns.91 It is mainly caused by the absence of NC-derived enteric ganglia along a variable length of the intestine, resulting in intestinal obstruction and massive dilation of the proximal bowel. HSCR can be classified into Short - segment HSCR (S-HSCR) and Long-segment HSCR (L-HSCR) due to incomplete penetrance of the disease causing mutations that affect variable length of the intestine.91,92 Ten HSCR susceptibility genes have been identified in humans, including RET, SOX10, PHOX2B, EDBRB, END3, ECE1, ZFHX1B, GDNF, NRTN and KIAA1279.93 Surgical removal of defective bowel is the standard treatment of HSCR, however, for L-HSCR patients, only small portion of bowel was left that is not sufficient for intravenous nutritions absortion.
Recent study from Fattahi & colleagues revealed an alternative therapeutic approaches for HSCR.94 They established a differentiation protocol to generate enteric progenitors from human PSCs effectively by addition of retinoic acid (RA) and that could be further differentiated into functional enteric neurons. In vivo transplantation of hPSC-derived enteric progenitors into chick embryos and adult mouse further confirmed their enteric NC identity, as demonstrated by their ability to invade to the gut region. Importantly, engraftment of these iPSCs induced ENS progenitors increase survival rate of HSCR mouse model harboring mutations in Ednrb. Finally, a candidate drug, pepstatin A, was identified after a small-molecule screen and demonstrated to restore aberrant migration of enteric NCCs in Ednrb -null mutants (Figure 2).
In the past two decades, advances in molecular biology and functional genomics have provided a greater understanding of how various molecules expressed at different stages of NC development are functionally connected together to form a gene regulatory network that underlies the process of NC progenitors formation, migration and differentiation. These basic developmental studies are essential in several aspects. First, it provides insight into what genes are evolved leading to the emergence of NCCs essential for the evolution of vertebrates by modelling the function of the head to facilitate the shift of ancestral vertebrates from filter-feeders to active predators. Second, increasing evidence suggests that genes involved in NC development are often dysregulated in NC-derived tumors such as melanoma and neuroblastoma. Understanding the molecular mechanisms underlying NC formation, migration and differentiation could gain insight into the initiation and progression of tumors with a NC origin. Third, it informs us the identity of extrinsic and intrinsic molecules required for the expansion and maintenance of hESCs/iPSCs-derived NCCs or iNCs and adult-derived NCSCs as well as directing their differentiation into specific lineages for cell and tissue replacement strategies. Fourth, it provides gene signature to evaluate the identity, regulatory and developmental state of in vitro- derived NCSCs/progenitors by direct differentiation from PSCs or lineage reprogramming from somatic cells.
Although promising results have been demonstrated for the developmental and therapeutic potential of NCSCs/progenitors in animal models, there are some key issues that need to be addressed. Current differentiation protocols using SOX10-EGFP reporter tend to generate hNC precursors with cranial/anterior identity. Recent studies identified a new surface marker, CD49D to mark SOX10+ NC precursors with vagal identity following addition of RA into the differentiation medium.94 Consistently, similar RA treatment promotes specification of trunk NCC progenitor sympathoadrenal (SA) lineage which could be further directed to SA cells in the presence of BMPs.95 Based on the markers expression in this study, it is not clear the efficiency of trunk NCSCs generation using this differentiation regime and other lineages such as melanocytes and neurons were present in the culture. Further optimization of the differentiation protocol and identification of unique surface proteins are crucial for the enrichment and purification of each NC-derived lineage respectively. Given the limited amount of data on hNC development, it is necessary to perform transcriptome analysis for the establishment of gene signature in each NC -derived lineage. This information should further inform the conditions required for the differentiation of hESCs/iPSCs into specific NC derivatives and their maintenance.
Although the ability to generate patient-specific hiPSC-derived NCs is a powerful platform for modeling of neurocristopathies to study its pathogenesis, these NCCs and their derivatives carry gene mutations and are not ideal for therapeutic applications. Genetic correction of mutation in hiPSCs can now be carried out using CRISPR/Cas9 to restore the normal function of the tissue specific cell type derived from hNCCs. Even though we have obtained a highly enriched and genetically corrected NC progenitors for a specific lineage, it is important to access the extent of integration in the disease animal model after transplantation to ensure the NC-derived cells are functionally stable in long term. On the other hand, non-viral approach such as plasmid-driven strategy and small molecule compounds to mediate lineage reprogramming of somatic cells into iNCs is important to prevent tumorigenesis upon transplantation into the host. Altogether, these endeavors will ensure hNCCs and their derivatives are clinically safe and functionally integrated to the host tissue for the disease treatment.
Because of their broad developmental potential and accessibility, NCSCs/progenitors have attracted increasing attention in the field of developmental biology and stem cell. With the advent of omics technologies and availability of high throughput drug screening platform, it is anticipated that research on understanding the biology, genetic regulation of hNCSCs and their derivatives as well as identification of drugs to restore the functions of patient-specific iNCs will be immense in the forthcoming year.
Jessica AI Liu is supported by the postdoctoral fellowship scheme in the University of Hong Kong and grant from the Research Grant Council and University Grants Council of Hong Kong (GRF/RGC_17110715).
Work in the Cheung Laboratory is supported by grants from the Research Grants Council (ECS_27100314, X_HKU709/14 and GRF/RGC_17110715).
The author declares no conflict of interest.

©2016 Liu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.