Journal of
eISSN: 2475-5540


Review Article Volume 1 Issue 2
PPG Ci
Correspondence: Alcyr Alves de Oliveira, Universidade Federal de Ci, Tel (51) 33038826
Received: October 25, 2015 | Published: March 24, 2016
Citation: Oliveira AAD, Sánchez JPB, Hurtado JDC. Neural stem cell transplantation and mechanisms for functional recovery. J Stem Cell Res Ther. 2016;1(2):59-71. DOI: 10.15406/jsrt.2016.01.00012
Neural stem cell transplantation has demonstrated its therapeutic potential in many neurological disorders. The long-thought prime mechanism of action was the replacement of cells lost to injury or neuro degeneration. Now, more and more evidence has provided insight into other bystander mechanisms through which these cell grafts could bring about a functional and structural restorative benefit. Their role in immunomodulation, neurogenesis and brain plasticity, as well as their capacity to secrete constitutively neuroprotective factors, open interesting doors to new frontiers in therapeutics that go beyond cell substitution. The purpose of this review is to outline the factors, both host and graft dependent shown to mediate these new mechanisms of therapeutic action posterior to NSCs introduction into a pathological host environment.
Keywords: neural stem cells, stem cell transplantation, neurogenesis, neuronal plasticity, cellular factors, host derived, neuroprotection, neuro immunomodulation, growth factors, antigens, differentiation
CNS, central nervous system; NSCs, neuronal stem cells; EGF, growth factors; FGF, fibroblast growth factor; BDNF, brain-derived neurotrophic factor; SVZ, sub ventricular zone; SGZ, sub granular zone; MSC, mesenchymal stem cells; BrdU, bromodeoxiuridine; MCM2, mini chromosome maintenance protein2; PH3, phospho histone h; PCNA, proliferating cell nuclear antigen; DCX, doublecortin; PSA-NCAM, poly sialylated embryonic form of the neural cell; NeuN, neuron-specific nuclear protein; MAP-2, microtubule-associated protein 2; GFP, green fluorescent protein; GFPA, glial fibrillary acidic protein; FGF-2, fibroblast growth factor 2; RMS, rostral migratory stream; VEFG, vascular endothelial growth factor; HB-EGF, heparin-binding epidermal growth factor; SCF, stem cell factor; SDF-1, stromal cell-derived factor-1; UPA, urokinase-type plasminogen activator; ECM, extracellular matrix; CXCR4, cxc chemokine receptor 4; MHC, major histocompatibility complex; IGF-1, insulin-like growth factor; GDNF, glial cell line-derived neurotrophic factor; LIF, leukemia inhibitory factor; NGF, nerve growth factor; CNF, ciliary neurotrophic factor; TSP, thrombospondins; TrkC, tropomyosin receptor kinase c; FasL, fas ligand; Apo3L, apo3 ligand; IFN-γ, interferón gamma; TNF, tumor necrosis factor; TRAIL, tumor necrosis factor-related apoptosis-inducing-ligand; GITR, glucocorticoid-induced tnf receptor; LIF, leukemia inhibitory factor; STAT3, signal transducer and activator of transcription 3; CNF, ciliary neurotrophic factor; PD, parkinson disease; AD, alzheimer’s disease
It was not until Altman et al.1 findings back in the 1960s1 that the concept of adult neurogenesis in the mammalian brain began to be built on and, with it, a new vision on the adult central nervous system (CNS)ability for endogenous repair and intrinsic plasticity. Two specific neural niches for generating new neurons after development have been identified: the sub ventricular zone (SVZ),2,3 which extends along the wall of lateral ventricles, showing a rostral migratory stream (RMS) towards the olfactory bulb4 and the subgranular zone (SGZ) of the dentate gyrus of the hippocampus.5 In the SVZ, new granule and peri glomerular interneurons will be born, whereas the SGZ, which possess mainly a local activity, will generate new granular cells for hippocampal circuit renovation. Both zones are hypothesized to play an important role in brain function, cognitive flexibility and restoration after brain damage.6,7
An important amount of what we know now on this regard was brought about by the nineties and early twenties advancements in microscopy and immune histological techniques. It was possible to proof the persistence of this phenomenon throughout life, albeit its slight decrease with increased age,8 the regulation exerted by environmental and behavioral factors such as stress,9 drugs10 and exercise11 (among others) and very importantly, the existence of this process in humans as well.12 During this time, methods to isolate adult or embryonic neural stem cells by dissecting out adult or fetal tissue in order to culture, maintain, proliferate and differentiate them under specific artificial lab conditions (e.g. growth factors such as EFG (epidermal growth factor) or FGF-2 (fibroblast growth factor)) began to emerge.13 In vitro techniques such as neurospheres, monolayer cultures and oncogenetic modifications (to mention but a few) in conjunction with studies in vivo gave way to important advances in understanding the biological aspects and the mechanisms involved in the regulation of these particular cells. They proved the functional and synaptical integration within the adult brain of new-born cells into the preexistent circuits and highlighted the importance of an adequate timing and neurogenic microenvironment for differentiation and migration.14–16
In the following years, higher interest was casted on the potential regenerative therapy they could represent.17 Transplanting stem cells expanded in vitro into the developing or adult brain (grafting) provided better results than anticipated. Not only by greatly contributing to our knowledge of the development of the CNS, neuronal migration and the intrinsic and extrinsic factors involved in differentiation;18 but also by becoming a medium for gene therapy and repair in many diseases characterized by brain damage and neuro degeneration.17
Neural stem cells (NCS) are cells with self-renewal capacity and, depending on their origin (embryonic, adult) and intrinsic factors, with pluri or multipotent capability for giving rise to different CNS cell linages as well as other tissues´ cell types (contrary to what was thought for a long time),19 hence their promising use. However, because they have been subjected to different protocols in order to manipulate them, the results have been very diverse and in some cases even counterproductive.20 Notwithstanding, research on this field has progressed and nowadays, although NSCs cell therapy still continues under intensive scrutiny, its promising potential has attracted even more attention due to accumulative evidence that suggests its participation in restorative processes goes beyond the replacement of loss cells,21 encompassing as well immune modulation and neuro protection.22
The purpose of this article is therefore to review the mechanisms known so far to intervene in the graft-host interaction after NSCs transplantation, both at the molecular and cellular level, and that consequently lead to behavioral effects at a major scale. By dividing discovered mechanisms into endogenous and exogenous, we hope to synthetize published research and provide the reader with a clearer, up to date and more integrative vision of what is known to happen posterior to the introduction of these cells. For this purpose, we will make a general introduction to the topic, addressing key issues and concepts, and will subsequently embark on the issue at hand. It does not mean to be exhaustive but rather to outline the different processes thought to occur within the brain, within the neuro pathological environment, posterior to the exogenous NSCs grafting and their functional consequences.
Neural transplantation of neural stem cells
The clinical use of neural transplantation is currently being explored as a strategy to promote recovery and repair of brain dysfunction provoked by pathologies such as Huntington’s, Alzheimer's, amyotrophic lateral sclerosis and Parkinson’s disease.23 Other neurodegenerative or traumatic conditions of CNS, such as the autosomal neurodegenerative disorder and spinal cord injury follow next in the queue for clinical trials. Given this wide range of disorders, at present, many stem cell types and their derived progenitors are being used in cellular replacement therapies; among these, the NSCs are one of the ideal strategies used in these conditions. The effectiveness comes associated with their link with the development of the CNS. Their origin in the neuro ectoderm provides them with a particular molecular composition: SOX1 and Oct4. SOX1 is a transcription factor that takes part in neural determination and differentiation during the early stages of the embryonic CNS and shows a synergic interaction with other important transcriptor factors during neurogenesis, such as the III POU class. Together, they modulate the expression of nestin (a type VI intermediate filament protein of special importance in cell division) and therefore are of vital importance in the regulation of neural primordial cells.24 Nestin and SOX1 are now recognized markers for NSCs in vitro and in vivo.25,26 Another important POU family´s transcriptor factor, the Oct4, give these cells the property of pluripotenciality and high capacity of differentiation,27 given its involvement in the self-renewal capability of undifferentiated embryonic stem cells. Stem cells are claimed to have the ability to develop phenotypes of different brain cell types (neuronal and glia) and, what is more, to migrate after grafting.28,29 Some types of grafted cells have shown a selective migration to lesion sites, which is a very convenient characteristic. All of the traits aforementioned in association with their capacity for secreting neurotrophic factors and modulating different mechanisms of regeneration and immune reactions30 have made them a very suitable choice. Today, the therapeutic use of neural stem cells covers not only the “recovering or substituting cells lost to injury, disease or physiological turnover” explanation; but also includes a guided cell differentiation effect and the stimulation of host plasticity and its endogenous mechanisms for repairing. A fact that has rendered the initial idea of neural transplantation as a technique to “only replace dead neurons with implanted healthy neurons” obsolete.
Pre-clinical studies using animal models have demonstrated positive behavioral effects with the use of neural stem cell transplantation in pathologies with cholinergic dysfunction,31–33 movement disorders,34–36 stroke,37,38 spinal cord injury,39–41 brain tumors,42–44 to name a few. Also, clinical trials have demonstrated symptomatic relief in humans that had undergone this cellular replacement therapy.45,46
However, it is still necessary to resolve many challenging issues associated with the use of this strategic therapy. The best strategy to control and regulate the differentiation and cellular growth is yet to be established, conjointly with ways to promote migration and to improve and achieve an effective functional integration within the host.47
Sources for neural transplantation
Different sources of NSCs are in use in the present age,48 which is better or brings the greatest benefits is yet a matter of great debate.
Despite the potential advantages in their use, many ethical and practical restrictions come with the translational clinical application of neural transplantation with human embryonic stem cells (blastocyst and embryonic CNS-derived). On one hand, in several countries, the use of embryonic human tissue is forbidden or restricted and there are numerous moral and religious principles that usually impose firm opposition to the use of unborn human material, even for therapeutic ends. On the other hand, technical difficulties involved in the use of embryonic material (e.g. viability and hetero/homogeneity of donated material) represent serious problems for its widespread use. While there are problems with dissecting the embryonic material and ways to maintain the cells alive, other issues such as the amount of brains needed for each surgery, the immunological rejection post-transplant and the propensity to form teratomas represent barriers to overcome. Stem cells obtained from adult brain niches such as SGZ or SVZ have a proved capacity for plasticity49 but have the limitation of a limited availability and therefore difficulty in obtaining sufficient numbers for clinical application.
Other less ethical constricted sources (blood, fat, skin, bone marrow, umbilical cord, etc.) come with the additional requirement of differentiation towards the neural linage, seemingly possible through specific in vitro manipulations/protocols and in vivo neurogenic cues.50 These adult mesenchymal stem cells (MSC) provide a promising exciting solution to overcome immunological drawbacks as an autologous transplant becomes possible, they per se have shown interesting immunomodulatory properties51 and as recently shown by Feng et al.,52 neural stem cells can be produced from them. Still, further evidence of their true therapeutic potential and standardized protocols are required.
Another alternative involves the possibility of generating a stock of expandable cells. They could be studied and analyzed in the laboratory and be safely prepared to supply for surgery at any time required, solving the issue of few limited number of donors. This is done by creating immortalized neural stem cell lines53,54 via the introduction of an oncogene using a retroviral vector or the fusion with tumor cells.55 The procedure enables the cell to proliferate in cell culture under specific artificial conditions and to differentiate into mature cells after implantation in the adult brain. The stumbling block: reported tumorigenic potential and unstable genotype.
All sources have been tested through different protocols and in different neurological conditions and thereupon results of varied value have been yielded; all possess advantages, disadvantages, and inherent technical and clinical challenging issues. The ongoing and future research will shed light on the alternative of higher beneficial for specific pathological conditions.
Identifying and tracing NSC and their progeny
A crucial step in the success of stem cell therapy is the ability to visualize, identify and track these cells fate both in vitro and in vivo. Traditional dying, immune histochemical techniques and division and phenotypic markers (thymidine analogs; antigens for antibody identification) are being complemented today by a series of genetic assays which primarily induce the expression of molecules in subclasses of stem cells and their progeny.56
The formation of in vitro floating aggregates (neurospheres) after exposing dissociated NSCs to growth factors has provided a way to study, screen and select stem cells to be grafted. These three-dimensional structures contain both undifferentiated and differentiated cells and subsequent marker identification and rigorous clonal and subclonal analysis should be carried out in order to analyze NSCs multipotency or self-renewal potential and, when selecting, avoid picking a heterogeneous cell population for transplanting.57
Once transplanted, it is necessary to be able to tell exogenous cells from endogenous ones. PKH26, a stable and long-term membrane-binding fluorescent dye, long used for cell therapy tracking,58 has shown the possibility of transferring, both in vitro and in vivo, to non-labelled cells,59 thus creating the chance of mistaking host cells with transplanted cells and obtaining a misleading survival assessment. Therefore, techniques that are more reliable are frequently used now. Among the nucleotide analogs, BrdU (bromodeoxiuridine) is the most used. Initially developed as a strategy to assess the proliferation index of tumors, it rapidly became a marker for neurogenesis in situ because of its properties. By integrating into the DNA during the S-phase of the cell cycle, BrdU came to be recognized as a marker for DNA synthesis, a process majorly occurring during cell division. It can be readily identified by an antibody directed against the DNA strand containing it and through its co-labeling with other cell markers (see below), it has allowed phenotypic analysis. However, related toxicity, mitogenic, transcriptional and translational effects and, although to a lesser extent, the possibility of its incorporation through other cell processes (such as DNA repair, abortive cell cycle reentry and gene duplication without cell division) have made it primordial to design rigorous protocols to control its pulsings. This will help in avoiding misinterpretation of repairing or dying cells with new born ones.60
From various studies over time, nowadays it is recognized a natural temporal and spatial expression of a sequence of cell markers (antigens) throughout the process of division and differentiation of stem cells into mature ones (Table 1), antibodies developed against them have allowed their identification and so that of the cells they are particular of . These are complemented by the generation of transgenic animals, through constitutive or induced (with tamoxifen or doxycycline) recombination (CreER lines), and virus-transmitted marker genes that create indelible artificial fluorescent cell marks (green fluorescent protein (GFP), cyan fluorescent protein (CFP), DsRed, mCherry, and tdTomato, among others) of different colors, timing of expression or localization).56
Marker |
Nature |
Expression |
|---|---|---|
Proliferating cells |
||
Ki67154 |
Nuclear protein |
During most phases of the cell cycle (except for G0). |
PCNA- proliferating cell nuclear antigen155 |
Nuclear protein - subunit of DNA polymerase-delt |
During all phases, highest in early G1 and S-phase |
PH3 - phosphohistone H3156 |
Histone octamer |
During the late G2 and M phase |
MCM2- Minichromosome maintenance protein 2157 |
Nuclear protein- component of the pre-replication complex |
Begins at early G1, all phases |
Precursor cells (stem cells and progenitor cells) |
||
Nestin26 |
Type VI intermediate filament. |
Diving cells during early stages of CNS development and in adult NSCs. |
Musashi 1154 |
Neural RNA-binding protein |
Highly in neural fetal and adult NSCs |
Sox2 159 |
Transcription factor |
Especially Adult NSC. Stem cells and precursor cells during development too |
Oct3/4 160 |
Transcription factor |
Embryonic stem cells and progenitors. |
Immature cells |
||
DCX –doublecortin 161 |
Microtubule-associated protein |
Migrating neuroblasts persist in young post mitotic neurons. |
PSA-NCAM - polysialylated embryonic form of the neural cell |
Homophilic binding glycoprotein |
|
Tuj-1163 |
Neuron-specific class III beta-tubulin |
Early postmitotic and differentiated neurons and in some mitotically active neuronal precursors. |
Neuro D 164 |
Transcription factor |
Early cells of the neuronal lineage. Precedes PSA-NCAM. |
TUC 4 -TOAD/Ulip/CRMP 165 |
Protein family expressed in the neuronal growth cone. |
Migrating mitotic cells and early immature neurons. Co-express with DCX and PSA-NCAM |
Vimentin 166 |
Intermediate filament |
Radial glia and immature |
Mature cells |
||
NeuN-Neuron-specific nuclear protein 167 |
Nuclear protein |
Most neuronal cells. |
Prox1 168 |
Transcription factor |
Postmitotic young and mature neuronal cells in the DG |
MAP-2 a and b isoforms169 |
Microtubule-associated protein |
Neuronal cells |
S100beta166 |
Calcium-binding protein |
Astrocytes |
GFPA- Glial fibrillary acidic protein 170 |
Intermediate filament |
Mature astrocytes, adult neural stem cells |
Table 1 Immuno histological markers for Stem Cells and their progeny.
All these techniques complement each other. Neither of them lacks limitations and neither is completely suitable for all kind of studies; it is up to researchers, by being familiar with these methods advantages, disadvantages and methodological limitations, to choose those of higher value for their specific research objectives and adequate their results interpretation to those.
The success in using cellular replacement therapy in many traumatic and neurodegenerative diseases involves three independent mechanism that need to be studied and understood more deeply. These are the survival of injected cells, their ability to migrate to the site of injury, to influence the underlying microenvironment and their capacity to be integrated into the host´s neuronal networks.61 A cross talk between grafted cells and host cells is thought to mediate these and so the functional effects seen with this therapy.
It is well-known that not only one mechanism is primarily responsible for recovery; different levels of reorganization may occur in different graft paradigms, neural systems and time intervals which might, in turn, exert an influence that could have been retarded without grafting. Some mechanisms such as chronic secretion/release of neuro chemicals into the neuropil as a response to grafting, or the reconstruction of the host brain circuitry as a process of self-repair and reciprocal reinnervation might play crucial role in graft effects,62 which can range from deleterious to fully reconstructing and neuroprotective. Besides, migration or other mechanisms involved in host-graft communication during the initial moments after grafting may trigger irreversible processes that promote plastic changes in the host brain and reconfigure its cell projections. Processes such as formation of glial scaffolding for migration and differentiation guidance63 and trophic factor release64 would be of vital importance therein.
Thus, neural stem cells grafted into the damaged brain may promote the activation of one or more of these mechanisms and, at some stage, interact with the host cells to exert effects leading to functional recovery. As it is, only a glimpse of what actually happens is known and further investigation on this matter will be of crucial importance in revealing the whole panorama, but it is meaningful to summarize and stand out key aspects of this process.
Albeit, when talking of intermediating action mechanisms, a conceptual division can be drawn (endogenous versus exogenous), the reader should be bear in mind that as they interplay in complex and reciprocal ways, a truly functional separation is more difficult to be made. Table 2 resumes this conceptual dichotomy and (Figure 1) represents it schematically.
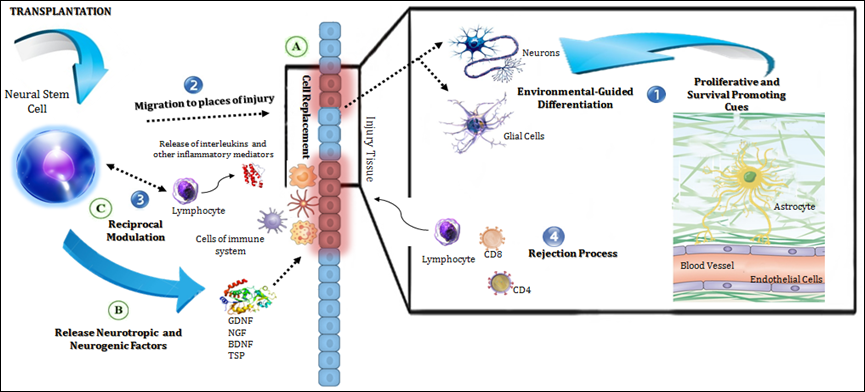
Figure 1 Mechanisms of action of transplanted NSCs in the host.
The diagram illustrates in a general manner the endogenous (in numbers) and exogenous (in letters) mechanisms of action of NSCs grafts.
Endogenous mechanisms:
Exogenous mechanisms:
Mechanisms of Graft Action |
|
|---|---|
Endogenous (host dependent) |
Exogenous (graft dependent) |
Proliferative and survival-promoting cues in the host environment |
Cell replacement and functional integration |
Chemoattraction to sites of injury |
Endogenous neurogenesis boosting |
Host immune system influence |
Exogenous immunomodulation |
Graft rejection processes |
Production of neurotropic factors and circuit rewiring |
Table 2 Endogenous and Exogenous Mechanisms Mediating NSCs Graft Actions
Endogenous mechanisms (host-dependent)
When the host exerts an action triggered by the implantation or the host environment per se has an influence on exogenous stem cells function. This is crucial because as we know, not just the genotype governs cell function, external cues also intervene and mediate important aspects of cell behavior, and ergo the host environment is of prominent relevance for cell therapy.
Environmental signals for differentiation and survival: Today it is clear that the neurogenic niche actively participates in stem cell maintenance, activation and differentiation.65 Surrounding blood vases, astrocytes, cerebrospinal fluid and a complex set of extracellular molecules secreted by the cells in the niche are the perfect set up for a dynamic interaction to occur within; here, cell to cell communication takes place and many diffused signals and molecules, soluble or embedded in the extracellular matrix and associated blood vessels, coact spatially and temporally for regulating stem cell biology.66 This is reflected by the fact that in-vitro cultured stem cells respond and are largely controlled by the neurogenic microenvironment of the host, where they might show different multipotency to that observed when cultured in vitro and vice versa.
Examples of this can be found. Stem cells obtained from the neocortex (non-neurogenic region) where they only develop into glial cells, show neuronal and astrocytic lineage differentiation when exposed in vitro to growth factors such as FGF-2.67,68 On the other hand, stem cells harvested from neurogenic zones show very limited neurogenesis when implanted into non-neurogenic locations.69 Cord blood derived NSCs respond to signals from an in vitro neural-like microenvironment, which promoted different phenotypes in accordance with different cell to cell intercommunications.70 Human skin-derived stem cells AC133(+) when engrafted in the mouse brain showed neuronal and abundant astrocytical differentiation,71 a phenomenon equally seen with neural progenitor specific types (Thy1 and Sca1 positive) derived from the murine bone marrow.72 In vitro techniques where skin derived cells where exposed to a medium containing postnatal hippocampal-astrocyte-derived signals produced stable neuronal cells with preserved physiological responses,73 highlighting the role that mature hippocampal astrocytes and their signals at the niche possess in neurogenesis, neuronal fate and survival and synaptogenesis.74,75 In a 6-OHDA Parkinson disease model, light stimulation of endogenous (transgenic) and optogenetically modified astrocytes (co-transplanted with embryonic neural stem cells) in the substantia nigra elevated bFGF secretion and, with it, a significant increase in dopaminergic differentiation of the transplanted cells was observed; this was traduced in a functional improvement measure by the apomorphine-induced rotational behavior test.76 Other factors present at the surrounding niche, such as the vascular endothelial growth factor (VEGF), the nitric oxide or the brain-derived neurotrophic factor (BDNF) have also been shown to modulate neurogenesis.77,78
Endothelial cells are crucial elements of this neurogenic stimulus; neurogenesis and angiogenesis have been tightly linked and influence each other reciprocally. As mentioned earlier, NSC reside in a vascular niche where intimate contacts are made with neighbor endothelial cells; this close relationship has been hold as an important regulator of endogenous NSC proliferative and differentiative properties, together with the formation of new vessels. Therefore, it is not out of line to think they may as well play an influence in transplanted NSC therapeutic effects. Literature on the latter is still a bit scarce; however, some studies conducted on the matter have pointed positively towards the role of endothelial cells in the maintenance of self-renewal and pluripotency capacity of transplanted stem cells. A study conducted with a mice model of stroke79 found an increased proliferation, survival and neuronal differentiation of stroke-induced NSC when co-transplanted with endothelial cells. Within a cell culture,79,80 these cells seemed to favor the differentiation to neuronal precursors (an effect greater than that observed with astrocytes co-cultures) and that depended, apparently, on physical contact as much as diffused mediators such as VEGF, BDNF, FGF-2 or IGF-1. Furthermore, when co-transplanted with endothelial and astrocytes, the NSCs showed better performance at improving memory ability in the water morris maze of a rat model of stroke.81 The metabolic, structural and trophic support these cells provide seems thus of decisive importance as they can promote the survival of the transplanted cells as well as their migration and neuronal differentiation within the sites of lesion.
Further evidence that local factors are determinant in cell fate comes from the fact that, independently of the brain site where stem cells were harvested from, the phenotype of their mature cells mirror those from the target region of implantation and not those from the origin. For example, stem cells from embryonic forebrain regions gave origin to glial and neuronal cells with specific morphologic features of targeted zones (striatum, hippocampus or cortex).82 Likewise, migration, axonal projection and connectivity of newborn cells are also driven by regional factors in the developing brain. Cells from grafts in the striatum send projections along the internal capsule down to the mesencephalon, whereas those from SVZ grafts do so through the RMS. Hippocampal grafts show local migration with integration into neighbor circuits.29 Alike biological features favors functional incorporation in host circuitries.83,84
New generated cells are indistinguishables from resident cells and, surprisingly, recent studies have shown that some NSCs could even fusion with local mature elements to form hybrids at a rate which varies between different cell subtypes,85 a phenomenon which may as well be dependent on microglial copresence and multiple ligand and molecules intercellular interactions.86
Migration to places of injury: An important role of the niche is ensuring the right balance between quiescent and activated stem cells under specific tissue conditions (maintenance, injury, disease), so when needed, stem cells could be recruited, activated and mobilized to the sites where they are most functionally needed.87
The capacity of stem cells to migrate parenchimally across great distances to places of injury in the brain is of major value for stem cell therapy88 and has been associated with processes of neuro inflammation, reactive gliosis and angiogenesis occurring at the injury site. The complex molecular mechanisms governing this mobilization and homing are yet being elucidated and just a tip is known.
After lesion, astrocytes, microglia and immune cells are activated (reactive gliosis); this leads to a change in cell to cell communication and ECM conformation and with them, the signals that control different biological responses such as regeneration and plasticity.89 Different chemokines, adhesion molecules and growth factors play a stem cells chemo-attractant and regulatory role, both during development and posnatally damaging conditions;90 in some cases, components that resemble stem cells niches are re-expressed and endothelial cells involved in processes of local angiogenesis interact and modulate NSCs behavior. This way, the conditions for their migration, survival and differentiation are set.89 It is worth mentioning that each type of CNS injury will alter ECM composition in a very particular way and so the array of signals the transplanted NSC will encounter when implanted will be disease-specific as well. This might account for the varied therapeutic results across different pathologies, to what extent, it is still uncertain.
Examples of this. In models of cerebral ischemia, FGF-2, Heparin-binding epidermal growth factor like growth factor (HB-EGF) and the chemokine stem cell factor (SCF) through its c-kit receptor, seem to be some of the trophic factors which stimulates neurogenesis in this condition.91,92 Hypoxia also mediates NSCs attraction to brain tumors, where they could be used as a tumor-targeted drug delivery therapy43,44 by up regulating stromal cell-derived factor-1 (SDF-1), urokinase-type plasminogen activator (uPA), and VEGF,93 factors also implicated in processes of angiogenesis during tumor growth and invasion.94 Other proteins (Laminin and tenascin-C) in the tumor-produced extracellular matrix (ECM) are also highly permissive for NSCs migration.95,96 Inflammatory chemo attractant SDF-1 through its CXC chemokine receptor 4 (CXCR4) also enhances proliferation, promotes migration and transmigration of quiescent NSCs towards an infarcted zone97 and of transplanted NSCs towards both spiral ganglion neuron-degenerated cochlear and immune-mediated demyelination microenvironments.98 NSCs intravascular-delivered have been able to reach ischemic zones within the brain via a transendothelial recruitment mediated by the chemokine CCL2 and its receptor CCR299 and endothelial adhesion molecules ICAM-1 and VCAM-1.100
This evidence supports the premise that the site of injury, through the creation of an environment with a chemoattractive gradient, plays a vital part in NSCs recruitment and further modulatory exogenous actions. As said before, each pathological disruption will be characterized by a particular ECM composition and lesion microenvironment and different molecular and biological interactions will occur in singular ways. Characterizing these microenvironments and their interactions would be critical for a further understanding of NSCs properties and therapy in each particular case.
Immune system modulation: The tight relationship between NSCs and immune system is no longer a presumption, more and more evidence piles up supporting the mutual regulatory system these cells engage in. Having this in mind, it is no surprise that factors released by immune cells have an impact on NSCs behavior and self-renewal capacity, phenotyping and progeny survival; in fact, as addressed before, many inflammatory signals serve as NSCs attractants and immunodeficient mice have shown impaired neurogenesis. This effect could be both positive or detrimental at different stages of response and patterns of cell activation, as succinctly reviewed by Kokaia et al.101 By expressing toll-like receptors, NCSs have the potential to respond to different inflammatory soluble signals and change their fate.
Pro-inflammatory Th1 cytokines (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma), predominant in bacterial infections or ischemic environments for example, are mostly associated with negative effects on proliferation and neurogenesis, whereas Th2-related (IL-4, IL-10) cytokines possess the opposite effect. IL-4 for instance, facilitates microglia activation and with it, proneurogenic factors are secreted. Neurotrophic factors and interleukins secreted by migroglia (e.g. insulin-like growth factor (IGF)-1, BDNF, IL-15) also show a protective profile.102 Conversely, by shown in a murine model of cortical ischemia, Glucocorticoid-induced TNF receptor (GITR) activation on CD4+ t cells leads to a stronger inflammatory response at the site of lesion with a consequent apoptosis-related reduction on the number of endogenous stem cells/progenitors recruited.103 In some cases, such as multiple sclerosis, oligodendrogenesis is of therapeutical value; high quantities of INF-gamma have shown detrimental effects on this process, whereas low-doses in conjunction with IL-4 signal, have proven to promote it.101 Kinin-B2 (bradikinin) is also involved NSCs differentiation, it favors neuronal linage over glial linage differentiation and have displayed anti-inflammatory processes (through astrocytic action and decreased TNF-alpha production my activated microglia) within the brain at later stages of ischemic injury.104Rejection process: The brain, traditionally considered an immunologically privileged site for transplantation,105,106 is no longer held as that privileged. Despite not exhibiting frequent flow-blown graft rejection processes, some rejection-like reactions have been reported. In a subset of patients with Huntington´s disease, who were implanted with fetal grafts, biological signs of alloimmunisation to donor antigens (anti-HLA antibodies) were observed.107 In a rat model of Parkinson disease, xenotransplanted neural progenitor cells elicited a coordinated immune response in the host at different time points, which ranged up to 60 days post-transplantation.108
The understanding of the rejection processes involved in neural transplantation can provide fundamental insights into the handling of foreign antigens in the brain and the most efficient way of using neural stem cells to obtain better results. It is addressed here because it is a host response that might influence to some extent NCS therapy results. As an example, a modified dopaminergical neuronal xenograft lacking major histocompatibility complex (MHC) molecules survived in vivo for a longer period when compared to a wild-type xenograft.109
The major source for graft rejection is the recognition of the MHC I and II alloantigens present on the surface of cells. Acute or immediate, withal chronic or long-term rejection reactions, are mediated by CD8-positive and CD4-positive T-lymphocytes, which recognize these antigens in exogenous cells. Other minor H antigens can trigger this rejection response too, at a slower pace though.110 Although stem cells and their differentiated progeny have been reported to express MHC antigens after exposure to inflammatory cytokines,111 these stem cells display low levels of immunogenicity112 and require, if it indeed does, only temporary immunosuppressive cotreatment when transplanted.113 What seems curious is the fact that albeit not generating a strong immunological rejection, the host immune system is indeed aware of the grafted cells. This is showed by the formation of aggregates of mononuclear cells surrounding grafted cells nearby blood vases,113 by the fact that certain brain inflammatory conditions could lead to the expression of different immunoestimulatory antigens on the NSCs114 and an elevated expression of IL-1β, IL-4, and IL-6 in response to transplantation.115 The functional reason behind these perivascular clusters is still elusive, but digging deeper into this might reveal further interesting facts of the immunomodulatory properties (see below) and tropism of NSCs. Inner technical and traumatic variables during grafting also have been evaluated as possible sources of rejection; none has showed a bullet-proof evidence.113
As such, there is still no consensus regarding immunosuppression.116 Some studies have pointed out longer cell survival with different protocols (cyclosporine alone or in combination with methylprednisolone) in phylogenetically discordant transplants,117,118 with detection of transplanted cells up to the forth month post-transplantation, and a study blocking IL-6 production reduced immune recruitment and promoted neuronal differentiation in a similar fashion to Cyclosporine A.115 Some other studies have failed to prove a beneficial effect.119–121
It is worth considering that some studies have found that immunosuppressive medications could alter neural precursors proliferation capacity,122,123 an effect that, although failed to be replicated in a in vivo model,124 deserves further research.
Exogenous Mechanisms (graft dependent)When the implanted cells have an action of their own.64 The actions mediated by these cells surpass the cell-turnover function, long-hold as prime recovery mechanism, and now incorporate other bystander mechanisms of neuroprotection, immunomodulation and neuroplasticity. Through them, the NCSs prevent further tissue damage, rescue degenerating host cells and influence revascularization and processes of neuroregeneration.
Immunomodulation and neuroprotection: As seen before, immune and stem cells engage in a rich talk and form a close network that persists into the adulthood.104 This close relationship mediate the NCSs´ protective (to some extent) and immunomodulatory effects seen with this therapy, which ultimately safeguards the brain from inflammatory damage. All CNS pathological disorders are related, at different degrees, to a particular inflammatory process, hence NSCs, by expressing and secreting different factors that will affect immune behavior and repairing systems, will ultimately modulate different dysfunctional mechanisms and potentially guarantee their own survival and functional integration into the host neural circuitry.
Many researchers125–127 had acknowledged that NSCs have a direct action on the immune system by participating in the immunosuppression of macrophages, dendritic cells and T cell activation and proliferation. In vitro, this suppression has been related to nitric oxide and prostaglandin E2 production.128 Additionally, the release of growth factors such as Neurotrophin 3 (which modulates myelination and development of CNS) participate in the regulation of the Th1/Th2 balance (through its tropomyosin receptor kinase C (TrkC)) and processes of neuroprotection, remyelinization and neuronal replacement.129,130 Finding perivascular cuffs of undifferentiated NSCs, reactive astrocytes, endothelial cells and T cells after intravenous injection of NSCs in a model of chronic neuroinflammation, and the proapoptotic action of these surrounded NSCs on blood-derived Th1 cells, prompts towards a potential major putative therapeutic NSCs mechanism on chronic neuroinflammatory diseases such as sclerosis multiple.131,132 NSCs expression of death ligands (FasL, TRAIL and Apo3L) and the secretion of factors (nitric oxide, IFN-γ, glial cell line–derived neurotrophic factor (GDNF) and leukaemia inhibitory factor (LIF)) have been hold responsible for this.101 The process of antigen presentation and so T-cells activation also seem impaired by means of LIF production. In an experimental model of autoimmune encephalomyelitis, exogenous NCSs injected subcutaneously, hindered the activation of antigen-presenting dendritic cells in lymph nodes and so that of T-cells.127 Release of IL 4 and IL 10, and the participation of the latter in remyelination processes,133,134 also partake of immune regulation and neuroregeneration by the NSCs. In late phases of spinal cord lesion, transplanted cells have provoked a shift in the cytokine profile and fewer inflammatory cell recruitment. In fact, NCSs implantated in a severely contused spinal cord, during the subacute phase, stayed undifferentiated and established modulatory contacts with peri-lesional phagocytic cells, which lead to a change in the local inflammatory cell repertoire and improvement of motor function. Models of ischemia have also shown down regulation of markers of inflammation, glial scar formation and neuronal apoptotic death by NSCs influence at gene level.135 Horie et al.136 identified that the transplantation of NSCs grown as neurospheres elevates the release of VEGF in animal models, a factor involved in neovascularization and perfusion in stroke, leading to a consequent functional recovery.136,137 In fact, transplanted embryonic NSC have been shown to protect endothelial cells against ischemic-related death80 by means of, both in vitro and in vivo, VEGF-related vasculotrophism and downstream activation of the phosphatidylinositol 3-kinase (PI3 kinase)/Akt pathway. Data that supports a bidirectional influence of these two cell types in physiological and pathological conditions. Interestingly, interleukin 6 (a proinflammatory cytokine involved in the pathogenesis of several neurological disorders and associated with lower NSCs proliferation) prove to be neuroprotector in the ischemic brain after reperfusion via restoring the activity of STAT3. This signal transducer and activator of transcription 3 promote gene expression during angiogenesis and reperfusion after an ischemic event.138,139
Neurotrophic activity and circuit rewiring: The constitutive capacity of stem cells to produce neurotrophic factors and other molecules characteristic of the developmental stage is well known. The fact that this trophic expression changes with differentiation and environmental conditions140 makes its full characterization in vivo pretty complex. Exogenous stem cells modify injury mediated trophic expression,141 which is also specific to the underlying pathological process.In vitro, NSCs promoted axonal outgrowth and showed a protective effect against glutamate-mediated excitotoxic damage through the secretion of GDNF, nerve growth factor(NGF), BDNF and Neurotrophin 3.142,143 Factors also involved, in conjunction with ciliary neurotrophic factor(CNF), in host axonal growth and functional improvements in models of spinal cord injury and neurodegenerative diseases.144,145 After improving NCSs survival by conferring them antioxidative properties, the paracrine factors released by them (VEGF, GDNF) in an animal model of intracerebral hemorrhage increased the survival of neurons within the striatum, reducing its the atrophic deterioration.146 In many conditions, the capacity to rescue neurons in peril and reshape surviving circuits is paramount. Embryonic NSCs and Immortalized neural progenitor cell lineRN33B when grafted in neonatal hippocampus and/or cortex produced regional-like pyramidal neurons which exhibit normal electrophysiological properties and made functional connections with appropriate neighbor and contralateral regions, integrating in the host circuitry.83,84 NCSs, through the secretion of VEFG, thrombospondins (TSP) 1 and 2 (normally secreted by immature astrocytes during development to promote synaptogenesis) and SLIT (important in axon guidance and cell migration), were able to rescue axonal transport and generate axon sprouting in a model of cerebral ischemia. The subsequent rewiring from non-lesioned places coincided with functional recovery.147 Similarly, hippocampal NCSs transplanted 2 days post-stroke in a photo thrombotic mice model, reduced the infarct size by a little bit over of 15% and provided functional recovery on the rotarod test and limb strength, the neuronal differentiation nearly one month post-transplant was confirmed by immune cytochemistry analyses.37 The differentiation towards the neuronal lineage within the ischemic region was also seen in a middle cerebral artery occlusion and reperfusion rat model.38 In a toxic animal model of Parkinson disease (PD), functional improvements were seen following the implanting within the striatum of olfactory bulb-derived stem cells,34 of which 50% showed neuronal differentiation at 8 week post-transplant; cells that have obtained similar results in a model of Alzheimer disease when transplanted in the lesioned hippocampus.32 Likewise, beneficial effects were obtained by favoring the dopaminergic differentiation of implanted NSCs within the substantia nigra.35,76 In models of AD. Immortalized stem cells MHP36 implanted at lesioned basal forebrain and hippocampus improved the performance on spatial learning and memory tasks following their implantation, subsequent migration to, mainly, the striatum, thalamus and basal forebrain regions and final neuronal differentiation.148 NCSs modified to over-express the gen coding for the enzyme choline acetyltransferase differentiated into neurons after migration to the hippocampus and striatum, and helped to recover the learning and memory deficits seen in AD.31 A last example of neuronal replacement comes from a model of spinal cord injury,149 in which functional recovery was linked to the presence of transplanted NCSs and their neuronal differentiation and posterior formation of synaptic connections with host neurons.
Endogenous neurogenesis boosting: After an injury, endogenous stem cells can be recruited in order to compensate for tissue loss, a compensation of tissue-specific and age-dependent regenerative potential. Unfortunately, in most cases, this backup system is insufficient to restore function completely.150 A continuous aggression and progressive cell loss could affect, both directly and indirectly, NSCs endogenous niche, and with it, the whole restorative capacity. If the niche can affect transplanted cells behavior, the opposite is also possible. The use of exogenous NSCs comes in as a therapy with the cellular ability to recover and potentiate this endogenous repair mechanism. Studies in animal models of Alzheimer’s disease (AD), spinal cord injury, stroke and Parkinson disease contribute some evidence on this respect. In a mice model of AD, human NCSs transplanted bilaterally within the hippocampus produced an improvement on spatial memory that was related to an increase in the number of DCX positive in the dentate gyrus,151 a stimulating effect on endogenous neurogenesis also seen when transplanted in the lower spinal cord.152 A rat model of stroke also showed a higher number of proliferating cells and migrating neuroblasts in the SVZ in the group with the intrastriatal grafts when compared to that receiving a vehicle, even at 14 weeks post-transplantation. 153
Trophic and modulatory mechanisms reviewed previously may not only occur at lesioned sites, they can reach neurogenic niches to exert modulation. The, multiples neurotrophic and regulatory secreted factors would be the intermediaries of this boosting capacity.153–170
The therapies with NSCs hold a great potential for many neuro pathological conditions. Neural stem cells appear naturally endowed with the appropriate machinery required to express an otherwise silent genomic potentiality in response to an appropriate pattern of stimulation. The long-believed prime mechanism of cell substitution is slowly backing away from the spotlight to give way to new exciting bystander mechanisms by which neural transplantation could prompt a functional recovery. In order to be successfully translated into the clinical setting, a fully understanding of stem cells properties and the complexity of their interaction, upon transplantation, with the pathological cellular and molecular microenvironment they encounter, is needed. The mechanisms involved in a successful neural transplantation are multivariate, the exogenous and endogenous here reviewed interplay in manifold and heterogeneous ways. This host-graft reciprocal modulation impacts not only on differentiation, migration and survival of the implanted cells, but also mediates to a great extent the neuroprotective, immunomodulatory, neurogenic and neuroplasticity fostering-effect they are now recognized to possess. Partially conflicting in vitro and in vivo data on the role of the milieu-NSCs transplant communication warrant further research on the topic. More so, if we believe that different signaling molecules could act on completely opposed ends based on the particular pathological microenvironment they are embedded in. This insight will allow NSCs-based therapies to flourish in the new era.
This study was supported by the National Institutes of Health (1R15CA202656) and the National Science Foundation (1703570).
The author declares that there is no conflict of interest.

©2016 Oliveira, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.