Journal of
eISSN: 2373-4426


Case Report Volume 5 Issue 1
1Department of Obstetrics and Gynecology, Lincoln Medical and Mental Health Center, USA
2St. George's University, School of Medicine, Grenada
3Department of Pediatrics, Lincoln Medical and Mental Health Center, USA
Correspondence: Shadi Rezai MD , Department of Obstetrics and Gynecology, Lincoln Medical and Mental Health Center, 234 East 149th Street, Bronx, NY, 10451, USA
Received: June 21, 2016 | Published: August 10, 2016
Citation: Rezai S, Wilansky J, Gottimukkala S, Chadee A, Benamanhalli H, et al. (2016) Wolf-Hirschhorn Syndrome (WHS), A Case Report and Review of Literature. J Pediatr Neonatal Care 5(1): 00170. DOI: 10.15406/jpnc.2016.05.00170
Background: Wolf-Hirschhorn Syndrome (WHS) is a congenital malformation syndrome characterized by growth deficiency and varying developmental delays based on genomic deletions and characteristic facies. The majority of WHS cases are caused by a deletion of 4p16.3 regions on chromosome 4, which includes the Wolf-Hirschhorn Syndrome Candidate genes (WHSC1 and WHSC2). WHSC1 protein is required to inhibit DNA damage by regulating the methylation of histones. The diagnosis of WHS is established by detection of a heterozygous deletion of the Wolf-Hirschhorn Syndrome Critical Region (WHSCR) with 4p16.3 at approximately 1.4-1.9 kb from the terminus. The WHSCR region is restricted to a 165-kb interval in the 4p16.3.
Some of the associated structural defects, such as cleft lip and palate, occur more frequently in individuals with greater than 3 megabase (Mb) deletions. The quantity of base deletions is an important factor in explaining phenotypic variability WHS. The deletion size has a partial correlation to severity, but not a direct correlation, as the phenotypic affect may be more or less severely affected than would be expected by the size of the deleted megabase.
Most individuals affected by this disorder have distinctive facial features, such as a broad, flat nasal bridge and a high forehead described as the “Greek warrior helmet” appearance in which the eyes are protruding and widely spaced. Other facial features include a shortened distance between the nose and the upper lip (a short philtrum), a down-turned mouth, a small chin (micrognathia), poorly formed ears with small holes (pits) of flaps of skin (tags), a small head (microcephaly). Intellectual disability ranges from mild to severe in people with WHS. Compared to people with other forms of intellectual disability, their socialization skills are strong, while verbal, communication, and language skills tend to be weaker.
Case: We present the identification during routine obstetric sonographic imaging of growth restricted fetus affected by WHS with co-morbid oligohydramnios and abnormal prenatal obstetrics ultrasound studies, which showed micrognathia, echogenic kidneys and echogenic bowel, in addition to a three-week discrepancy between the estimated ultrasound age and the gestational age by last menstrual period (LMP), compatible with intrauterine growth restriction (IUGR). Prenatal genetic testing demonstrated female karyotype with a satellite chromosome on chromosome 4 short arm and mosaicism for an additional marker chromosome (47, XX, 4ps, +mar (8)/46, XX, 4ps). The prenatal diagnostic approach included fetal sonographic assessment and molecular cytogenetic investigation. After engaging in a multidisciplinary informed consent process at our institution, the patient chose to continue prenatal care and not electively terminate the pregnancy.
Conclusion: A 4p deletion on chromosome 4 can be associated with subtle chromosome imbalances in other chromosomes. For example, the t(4;8)(p16;p23) translocation may be undetected in routine cytogenetics, suggesting that this translocation may be more the most frequent translocation after the most common reciprocal translocation in humans, t(11q;22q). The extent of the Mb deleted within the 4p region located on chromosome 4 correlates with the severity of the WHS phenotype. Genetic counseling provides families with information on the nature and implications of inherited genetic disorders, as a means of facilitating shared health care decisions.
Keywords: array comparative genomic hybridization, chromosome deletion, del (4p) syndrome, dillan 4p syndrome, monosomy 4p, pitt-rogers-danks syndrome, pitt Syndrome, WHS pathogenesis, wolf-hirschhorn syndrome critical region, wolf-hirschhorn syndrome, wolf-hirschhorn (4p-) syndrome
ACGH, array comparative genomic hybridization; PRDS, pitt-rogers-danks syndrome; WHSCR, wolf-hirschhorn syndrome critical region; WHS, wolf-hirschhorn syndrome
Wolf–Hirschhorn Syndrome (WHS), also known as Wolf-Hirschhorn (4p-) syndrome, chromosome deletion, Dillan 4p syndrome, Pitt-Rogers-Danks syndrome (PRDS) or Pitt syndrome, was first described in 1961 by Americans Herbert L. Cooper and Kurt Hirschhorn. WHS is a contiguous gene syndrome caused by a partial deficiency of chromosome arm 4p, with critically deleted region in 4p16.3.1-4 “Its frequency is estimated as 1/50,000-1/20,000 births, with a female predilection of 2:1”.5 The disorder is caused by a partial loss of chromosomal material of the short arm of chromosome 4 (del (4p16.3)).1,2,6,7 The most common facial characteristics are the “Greek warrior helmet” like facies, such as a broad, flat nasal bridge and a high forehead. Midline defects in the brain, heart, palate and genitalia are also present.8,9 There is a distinct craniofacial phenotype (microcephaly, micrognathia, short philtrum, prominent glabella, ocular hypertelorism, dysplastic ears and periauricular tags), growth and mental retardation, delayed psychomotor development, difficulty in ambulation, often with ataxic gait, muscle hypotonia, and congenital heart defects. Less common characteristics include hypospadias, colobomata of the iris, renal anomalies, and deafness. Another characteristic feature is seizures where approximately 90% of individuals with WHS are affected.10
The size of the deletion varies among affected individuals; studies suggest that larger deletions tend to result in more severe intellectual disability and physical abnormalities than smaller deletions.11 According to Wieczorek et al.12 the cleft lip and palate, periauricular pits/tags and colobomata were missing in patients with deletions smaller than 9 Mb and congenital heart detects were absent in individuals with deletions smaller than 16 Mb, suggesting these defects are associated with larger deletions. According to Zollino et al.13 this study demonstrated that the deletion region varied from 1.9 to 3.5 Mb.14 All of these patients presented with characteristic facial appearance, growth delay, mental retardation, congenital hypotonia, and seizures. Different mechanisms have been reported to cause WHS, such as do novo deletions, familial translocations, and de novo translocations. According to Zollino et al.14 de novo 4p rearrangements more often occur in paternal meiosis whereas the unbalanced de novo translocations are maternal origin.15
Shannon et al.16 conducted a study that also happened to display data about life expectancy and cause of death among child and parent. From the moment patients are given bad news, such as their unborn child has a chromosomal disorder, parents ponder about their options of whether to continue or terminate the pregnancy. The life expectancy of the child is determined based on the information received by the parents. Knowing that the child has a chromosomal disorder and a poor life expectancy may lead to poor decision making later on regarding the health and medical decisions of the child. For example, resuscitation of the child and bonding amongst the child and parent. If the outcome if terminal, the difficult decisions regarding the child may suffer as a result. Therefore, speaking to parents about the necessary information regarding their child’s illness and what measures should be taken to prolong the life expectancy of their child is essential.16 This information will serve as a useful guide to clinicians when discussing life expectancy with parents. We, the authors’ of this case and issue, believe that if the parent’s attitude about their child’s life expectancy is optimistic, their child’s survival will be prolonged plus a stronger bond will be created between child and parent.
A 35-year-old gravida 3, para 2002 Hispanic woman underwent amniocentesis at 17 0/7 weeks of gestation because the fetus was at increased risk for fetal aneuploidy due to advanced maternal age (AMA) and abnormal maternal serum screening (Quad- AFP, HCG, Estriol and inhibin), Quad Screen positive for Down Syndrome, negative for neural tube defect (NTD) and trisomy 18. An amniocentesis is a procedure performed between the 15th and 20th week of pregnancy used to diagnose chromosomal abnormalities and fetal infections. In addition, an amniocentesis can determine the sex of the fetus. There are risks involved with an amniocentesis, such as fetal injury, for example, postural deformities if the amniocentesis is performed earlier than the suggested weeks, in addition to the risk of preterm labor, respiratory distress and chorioamnionitis. An amniocentesis demonstrated a female karyotype with a satellite chromosome 4 short arm and mosaicism for an additional marker chromosome (47, XX, 4ps, +mar (8)/46, XX, 4ps).17 Genetic counseling was provided to the couple with a description of the prenatal karyotype. The couple also participated in an in-depth discussion of the immediate and long term consequences that this genetic condition may present for their offspring. At 20 5/7 weeks of gestational age, the amniocentesis showed a baby girl with WHS.
There was no family history of congenital malformations. However, the patient’s second baby was a 7-year-old male with (mild to moderate) autism, who attends a special school. The patient was educated about the poor survival of the fetus on this genetic visit as well as on the pediatric visit. Patient reports that she was well aware of the poor survival of the fetus. The patient had multiple consultations from geneticists, neonatologists, and pediatricians, but the patient refused to terminate the pregnancy.
Multiple obstetric ultrasounds were performed throughout the pregnancy, which all focused on fetal abnormalities including: obstetric ultrasound at 27 weeks 6 days gestation, which showed micrognathia, echogenic kidneys and echogenic bowel Figure (1-3) as well as a three week discrepancy between the estimated ultrasound age and the gestational age by LMP, compatible with IUGR (Figure 4).

Figure 1 Ultrasound image from obstetric ultrasound at 27 weeks 6 days gestation showing: Echogenic kidneys bilaterally.
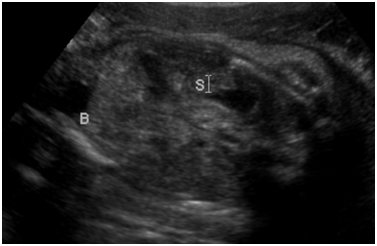
Figure 2 Ultrasound image from obstetric ultrasound at 27 weeks 6 days gestation showing: Echogenic bowel.
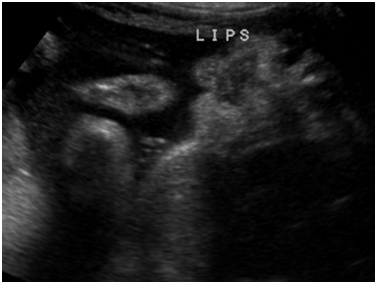
Figure 3 Ultrasound image from obstetric ultrasound at 27 weeks 6 days gestation showing: Suspected micrognathia.
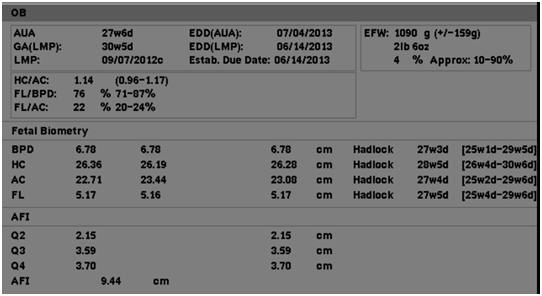
Figure 4 Ultrasound image from obstetric ultrasound at 27 weeks 6 days gestation showing: Three week discrepancy between the estimated ultrasound age and the gestational age by LMP, compatible with IUGR.
Patient underwent a 3rd repeat cesarean at 39 weeks delivering a malformed live baby girl, APGAR 7/8; Birth Weight: 2060 grams. The infant had micrognathia, microphthalmia, bushy eyebrows, an arched, flat bridged nose, mild hypertelorism, a small mouth with a high arc palate, a cleft upper gum margins, and low-set ears with bilateral cleft tragus. Neonatologists were present during the delivery the infant was immediately transferred to the neonatal intensive care (NICU) due to small for gestational age (SGA) and dysmorphic features, pulmonary hypertension (diagnosed by echocardiogram) and transient tachypnea of the newborn (TTNB). The postpartum care was unremarkable.
Follow-up of the baby
At 20 months of age living, the child is living with her mother, doing well, sleeps in own crib, receives continuous tubal feedings to supplement once daily oral consumption of baby food. To treat bilateral hearing loss and chronic superlative otitis media, tympanostomy tubes were placed. Recurrent seizures were treated with phenobarbital and keppra and physical examination showed frontal bossing and coloboma of optic nerve.
The incidence of WHS is estimated at 1:50,000 births, affecting females twice as often as males. It is due to a partial deletion of the short arm of chromosome 4 (4p-). About half of the patients with WHS have a de novo simple deletion of 4p16.3, while 40-45% of patients with WHS have an unbalanced translocation with both a deletion of 4p and a partial trisomy of a different chromosome arm.18 South et al.19 conducted two studies, which demonstrated that the rates of unbalanced translocations involving 4p in WHS is certainly higher than reported previously and is approximately 45%.19
In Giglio et al.20 demonstrated that an inversion polymorphism of the olfactory receptors (OR) region at 8p23 played a crucial role in the generation of chromosomal imbalances through unusual meiotic exchanges. As a result of this study, Giglio et al.20 conducted a study to determine whether OR-related inversion polymorphisms at 4p16 and 8p23 might also be involved in the origin of the t(4;8)(p16;p23) translocation”.21 “As a result, the study demonstrated that subjects with der22 had WHS, whereas subjects with der8 showed a milder spectrum of dysmorphic features.21” Results proved that two pairs of OR gene clusters were located very close to one another; both sets of OR gene clusters were located on 4p16 and 8p23. The study conducted by Giglio et al.20 confirmed that in 7 subjects (5 of whom represented de novo cases and were of maternal origin), including individuals with unbalanced and balanced translocations, breakpoints fell within the 4p and 8p OR gene clusters. In addition, the FISH experiments with bacterial artificial chromosome (BAC) probes detected heterozygous submicroscopic inversions of both 4p and 8p regions in all 5 mothers of the de novo subjects. “Heterozygous inversions on 4p16 and 8p23 were detected in 12.5% and 26% of control subjects, respectively, whereas 2.5% of them were scored as doubly heterozygous”.20
Wolf-Hirschhorn Syndrome can be diagnosed or suspected during the prenatal period by the following methods:
The present case provides evidence that an apparent 4p deletion on chromosome 4 can be associated with subtle chromosome imbalances in other chromosomes.25 It is important to educate the patient prenatally about the prognosis if she decides to continue with the pregnancy or if she decides to terminate the pregnancy. In addition, the NICU team should be informed and the NICU team should consult the patient upon diagnosis and during the pregnancy as needed. Furthermore, the NICU team consultation and patient evaluation is important if the patient chooses to have “comfort care only” upon delivery or if patient desires “everything to be done, including invasive procedures” for the newborn. All information should be given to the family regarding morbidity and mortality associated with infants with WHS. The NICU team should also be informed upon the patient’s admission for labor as their presence at the time of the delivery has been demonstrated to improve neonatal outcome as well as the pediatrics team.
Unfortunately, the treatment and management of individuals with WHS involves only supportive.26 The prognosis of WHS is poor. Frequently, a diagnosis with WHS is associated with fetal demise or infant death within the first year of life.27 Individuals who live past the first year of life, like the patient we describe in this case, have a slow, but constant physical and mental development. Within the first 2 years of life, 1/3 die within the first two years of life due to a heart defect, aspiration pneumonia, or from a seizure.27 Only symptomatic treatment is available. Specifically, to control the seizures, valproic acid is used for atypical absence seizures whereas benzodiazepines are used for status epilepticus, clonic and tonic-clonic types of seizures. In addition, surgery can be done to correct the cleft lip and/or palate.28
In conclusion, as stated above, genetic counseling is the most important factor when a making medical and personal decisions. Genetic counseling will be beneficial to the families of children with WHS. Parents can understand the associated risks of continuing the pregnancy to viability and what challenges they and their child will endure. The extent of the disease and the needs of the individual are based upon the following criteria:
The authors would like to thank Ms. Judith Wilkinson, Medical Librarian at Lincoln Medical and Mental Health Center Science Library for providing the reference articles.
None.

©2016 Rezai, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.