Journal of
eISSN: 2373-4426


Case Report Volume 2 Issue 5
Department of Pediatrics, Jawaharlal Nehru Medical College, India
Correspondence: Amar Taksande, Department Of Pediatrics, Jawaharlal Nehru Medical College, Sawangi Meghe, Wardha, Maharashtra, India
Received: August 15, 2015 | Published: September 4, 2015
Citation: Taksande A, Meshram R, Lohakare A, Singla A (2015) Variant of Shone’s Complex in a Child. J Pediatr Neonatal Care 2(5): 00094. DOI: 10.15406/jpnc.2015.02.00094
Shone’s complex is a rare cardiac anomaly consisting of multiple level of left sided obstructive lesions including supramitral membrane/ring, parachute mitral valve, subaortic stenosis, and coarctation of the aorta. We report a 5year-old girl child with a variant of Shone’s complex, associated with a patent ductus arteriosus and pulmonary hypertension.
Keywords: shone’s complex, mitral stenosis, coarctation of Aorta, patent ductus arteriosus
PDA, patent ductus arteriosus; SVMM, supravalvar mitral membrane; PMV, parachut mitral valve
Shone’s complex is a severe and rarely seen congenital anomaly. It is a congenital heart disease, consisting of multiple levels of left sided obstructive lesions including supravalvar mitral ring, parachute mitral valve, subaortic stenosis, and coarctation of aorta. When only two or three of these components are present, the incomplete form of Shone’s syndrome is diagnosed.1 This is a very rare malformation and a very few cases have been reported in literature.2 Here we present a rare case of variant of shone’s complex in a child which was diagnosed by echocardiography
A 5 year female child product of consanguineous marriage presented with history of breathlessness since 3month. She experienced progressive dyspnoea on exercise. On general examination, the patient was pale, the upper limb pulsation was good volume and palpable whereas lower limb pulsation was feeble and low volume. Pulse rate was 68/min in the upper limbs. Blood pressure level was 86/54mmHg, 90/58mmHg and 60/48 mmHg on the left arm, right arm and lower limbs respectively. Jugular venous pressure was normal. She had no peripheral edema. On cardiovascular examination, precordial bulge was not present. There was a heaving apex beat in the 6th intercostal area in the anterior axillary line. ON auscultation, first heart sound was loud with a mid-diastolic murmur at the apex. There was an ejection systolic murmur at the aortic area which was conducted to the carotids. Carotid Thrill was present. Other systemic examination was normal.
Investigations revealed hemoglobin value of 9g/dL, WBC count 4,000/cumm and platelet count was normal. Liver function test and renal function tests were normal. ECG showed the left ventricular hypertrophy. On chest X-ray there was a normal cardiac silhoutte and a normal lung parenchyma. Transthoracic echocardiography showed parachute-like mitral valve with a severe mitral stenosis (mitral valve size: 0.6 cm2, pressure gradient (Pg: 20 mmHg) causing obstruction to flow Figure 1. Aortic valve was bicuspid with a mild to moderate aortic stenosis (Pg: 40 mm Hg) Figure 2. Severe post-ductal coarctation of aorta with a diastolic tailing was seen on suprasternal view Figure 3. There was a Tiny mid muscular ventricular septal defect with left to right shunt. Small size patent ductus arteriosus with bidirectional shunt mainly left to right was present. Severe tricuspid regurgitation secondary to severe pulmonary hypertension (Right Ventricular systolic pressure = 84mmHg). Pulmonary artery was grossly dilated. There was mild left ventricular hypertrophy with a normal global systolic function and a normal ejection fraction. CT angiography revealed post ductal coarctation of aorta with patent ductus arteriosus with enlarged pulmonary trunk.
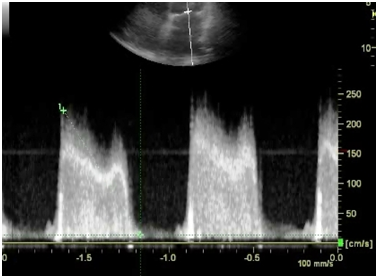
Figure 1B Continuous wave doppler studies demonstrated increased peak early (e) and late atrial (a) diastolic flow velocities, peak e-wave velocity is increased, 2.3 m/s.
Shone’s Complex (Shone’s Syndrome) described in 1963 is a rare congenital heart disease consisting of supravalvar mitral ring, parachute mitral valve, subaortic stenosis, and coarctation of aorta. It involves congenital left-heart obstructions at multiple levels. When only two or three of these components are present, the incomplete form of Shone’s syndrome is labeled. It is diagnosed most frequently in its incomplete form.1 The majority of patients with the incomplete forms have other associated congenital defects like patent ductus arteriosus (PDA), ventricular septal defect, bicuspid aortic valve and atrial septal defect.2,3 Our patient had parachute mitral valve, aortic stenosis and coarctation of aorta. There was other associated congenital anomaly like ventricular septal defect, patent ductus arteriosus, bicuspid Aortic valve and severe pulmonary hypertension were present. In Shone’s Complex, mitral valve obstruction during early embryogenesis is considered the first pathological event causes underdevelopment of the left ventricular cavity, which lead to various degrees of left ventricular outflow tract obstruction and aortic coarctation.
Supravalvar mitral membrane (SVMM), or mitral ring is circumferential ridge or membrane of connective tissue around the circumference of the mitral valve on the atrial side that may protrude into the valve opening and/or adhere to the valve leaflets. The ring may range from a thin membrane to a thick discrete fibrous ridge. Adhesion to the valve may impair opening of the leaflets causing mitral-valve inflow obstruction.1 Parachut mitral valve (PMV) is characterized by insertion of all the chordae tendineae into a single or fused papillary muscle group. The chordae are generally thickened, shortened and variable anatomy of the papillary muscles. However PMV also includes asymmetrical mitral valves having two papillary muscles, one of which is dominant and elongated, with its tip reaching to the valve leaflets. This results in a narrowing of the valve opening (mitral stenosis), restricted valve opening obstructing blood flow and, rarely, valvular regurgitation. Oosthoek et al.4 suggested that these morphological features distinguish a parachute-like mitral valve from a true PMV. Valvular and subvalvular aortic stenosis is a narrowing of the aortic valve and the channel below the aortic valve connecting the left ventricle to the aorta. Lastly, Coarctation of the aorta is a narrowing or constriction of the aorta, the large vessel that carries blood from the left ventricle to the body tissues.
Zucker et al.5 reported four cases of Shone’s complex among 12 520 echo studies in a series, which was diagnosed most frequently in its incomplete form. Takawira et al.6 documented an adult with Shone’s anomaly, bicuspid aortic valve, patent ductus arteriosus and pulmonary hypertension. Popescu et al.7 described a case of Shone’s anomaly in an adolescent girl who also had bi-atrial enlargement, bicuspid aortic valves and dysplastic mitral valves causing mitral stenosis and severe pulmonary hypertension. The cause for the pulmonary hypertension is multi-factorial and can be attributed to the mitral stenosis, PDA and long standing left to right shunt with elevated pulmonary vascular resistance. The presence of the coarctation of the aorta would also have worsened the left to right shunt. Many patients have severe lesions and present early in childhood as the patient becomes symptomatic by the age of 2 years. The common symptoms are breathlessness, tachypnea, nocturnal cough, poor feeding, failure to thrive, fatigue, and signs and symptoms of reduced cardiac output and heart failure. The child has recurrent episodes of wheezing and respiratory tract infections mainly due to pulmonary congestion and pulmonary oedema.3 Child had feeble lower limb pulsations as compared to upper limb pulsation which revealed the presence of a coarctation of aorta. Classifying cardiac lesions in infants is quite difficult, and accurate diagnosis is essential. The diagnosis of Shone’s complex requires an echocardiogram and a cardiac catheterization procedure. The echocardiographic findings revealed the features of variants of complete form of Shone’s complex. Clinical presentation and prognosis of patients with this anomaly are dependent on the complexity and severity of the different obstructive lesions.3–5 The management for this case would be significant reparable lesions, such as coarctation of the aorta or septal defects should be corrected early; mitral valve replacement should be deferred in favor of annuloplasty or reconstruction. Surgical repair may involve the replacement of the aortic valve in severe valvular aortic stenosis. A good outcome is possible in Shone’s complex patients, provided the early surgical intervention before the onset of pulmonary hypertension.8 Mitral valve repair along with supramitral ring resection is preferable over mitral valve replacement. Other surgical procedures depend upon presence of associated heart anomalies, which ultimately define late surgical outcome.
This is the very rare case of variation of Shone’s Complex in a child. It is very important to label all the cardiac abnormalities associated to this disease in view of a proper.
None.
The authors declare that there are no conflicts of interest.
None.

©2015 Taksande, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.