Journal of
eISSN: 2373-4426


Case Report Volume 4 Issue 5
1Pediatric Surgical Department, Obstetric and Children Hospital MITERA, Greece
2Radiology and Imaging Department, Obstetric and Children Hospital MITERA, Greece
3Anesthesiology Department, General Hospital EVANGELISMOS, Greece
4Plastic Surgery Department, Obstetric and Children Hospital MITERA, Greece
Correspondence: Stylianos Ypsilantis, Pediatric Surgical Department, Obstetric and Children Hospital MITERA, 2,Olympou 15234 Chalandri Athens, Athens, Greece, Tel 003-069-729-104-65
Received: January 27, 2016 | Published: May 17, 2016
Citation: Ypsilantis S, Vassiliadou E, Papaioannou G, Ypsilanti E, Savva A (2016) Total Excision of Congenital Mesenchymal Hamartoma of the Chest Wall in Newborn. J Pediatr Neonatal Care 4(5): 00152. DOI: 10.15406/jpnc.2016.04.00152
CT, computed tomography; NICU, neonatal intensive care unit; 3D, three dimensional; MRI, magnetic resonance imaging
Mesenchymal hamartomas, also called mesenchymomas, are very uncommon in the chest wall. First described by Nash1 in 1961, mesenchymal hamartomas represent a benign lesion that may involve the ribs, the sternum and the muscles of the chest cavity. They are associated with marked chest wall deformity that may cause respiratory distress in the neonate. The main challenge is the total excision of this tumor and the concomitant reconstitute of the affected tissues, since it usually occupies a large area of the thoracic wall and protrudes in the thoracic cavity. Radiologic imaging together with an expert multidisciplinary team of pediatric surgeon, anesthesiologist, cardiologist and an experienced NICU are of great importance in order to organize a successful surgical excision.2,3 During follow up orthopedic counseling for thoracic scoliosis may be required.4
Our young patient was a term female neonate, of 3150g birth weight, born with cesarean section due to history of fibroidectomy 2years ago. A round solid mass of 3-3,5cm diameter was detected antenatally with fetal ultrasound scan, adjacent to the lower third of the body of the sternum in keeping with lipoma. Post-partum clinical examination indicated a solid mass, of 3cm X 4cm X 5,5cm approximately, located in the inferior left parasternal area affecting the 7th and 8th costosternal synchondrosis Figure 1.
The neonate presented no signs of respiratory distress nor systemic failure. Plain radiograms shows the calcificated mass concerning to the 5th,6th, 7th ribs and the sternum with deterioration of the chest cavity Figures 2a&2b. Ultrasound of the area revealed a heterogeneous lesion, which casted significant acoustic shadow and was located symmetrically to the arches of the 6th, 7thand 8th left ribs. Multislice, low-dose CT scan was subsequently performed to clarify the origin, the extension and the configuration of the mass; 3D reconstructions were particularly helpful as they provided volumetric Figures suitable for preoperative planning Figures 3a&3b.
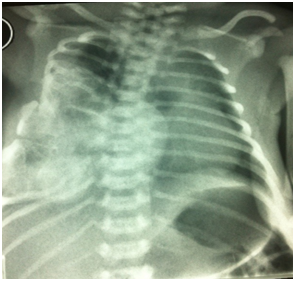
Figure 2a Plain radiogram of thorax. Is noted the large calcificated mass concerning to the 5th,6th, 7th ribs and the sternum.
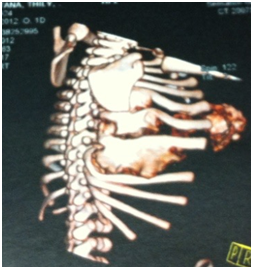
Figure 3b Is seen that the lesion concern at 5th, 6th and 7th right ribs. Scapula and the rest of the ribs aren’t affected.
Extrapleurar excision of the mass was performed under general anesthesia. The excision involved the 6th, 7th and 8th ribs on the left from the costovertebral junction to costosternal synchondrosis FIGURE (3). The pleura was left intact. The remaining cartilaginous ribs were anchored to the adjacent intact ribs by 0.2 polyglactin 910 absorbable sutures in order to minimize the size of the post-operative wall defect. The muscles were approximated using 0.3 polyglactin 910 absorbable sutures and “dura mater” substitution from bovine collagen absorbable membrane, stabilized by 0.4 polyglactin 910 absorbable sutures on the remaining tissues as a substrate for the muscle development. During the postoperative period, the neonate required NICU monitoring for 3days Figures 4a-4d. Paradox respiratory chest wall movement was noted during this time but it was not associated with any signs of respiratory distress of the neonate. The cardiorespiratory tests were normal and the neonate’s post-operative course was uneventful. She was dismissed on the 8th post-operative day in good condition. The tumor on histology presented chondroid tissue with endothelium lined vascular spaces, osteoclastic giant cells and osteoid in keeping with immature mesenchymal lesion.

Figure 3b Is seen that the lesion concern at 5th, 6th and 7th right ribs. Scapula and the rest of the ribs aren’t affected.
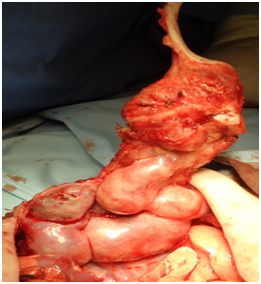
Figure 4b Intraoperative view of the lesion. Note that the affected tissues are solid and capsulated.
Follow-up MRI of the thorax performed two months later did not reveal any relapse of the mass. Thoracic scoliosis was seen on the AP plain film of the spine on the 4-month follow-up. The paradoxical respiration has resolved Figure 5. On the 6th month follow-up an appropriate scoliosis bandage was prescribed by the orthopedic surgeon Figure 6. Currently the baby is on orthopedic follow up for her scoliosis repair.
The main technical challenge in treating young patients with mesenchymal thoracic hamartomas is total excision of the tumor with careful restitution and closure of the thoracic cavity surgical defect6. If this mass is discovered on the antenatal sonographic fetal scan it does not present an indication to perform cesarean section delivery. A preoperative low-dose multislice CT scan with 3D reformats is necessary not only to confirm the benign nature of the lesion and avoid unnecessary needle biopsy but also to perform detailed surgical planning.7 The engendered defect can be covered by the remaining tissues, muscles and peritonea, mobilizing the residual cartilaginous ribs of the lower hemithorax.8,9 A slice of “dura mater” or Gore Dual mesh may be used as a support for the regeneration of the parietal muscles to ensure a more stable thoracic wall10. The use of double lined cement, referred by authors,11 for more rigid parietal reconstruction seems to be excessive and it is not advised. Paradox breathing movement of the affected hemithorax is not a significant postoperative problem for these neonates as it resolves in two to three months time. Furthermore this type of prosthesis shall be removed when the patient grows up. The only adverse to affront is the vertebra scoliosis of the thoracic part of the column. This postoperative late complication can be fronted initially by the use of appropriate bandage or surgical intervention for support the vertebral column.
There are different options to confront the lesion that varies from partial excision with12 or without13 radiofrequency thermoablation, with or without rib ablation, to total excision. There are various references for autologous implants or rib substitution but there aren’t references concerning to newborns. The extension of the surgical repair and the grade of excision/ablation depends on the grade of hemithorax involved, on the grade of respiratory distress and the operating room and postoperative intensive care support.14 The presence of a multidisciplinary group must be guaranteed.
Even if the recurrence of hamartomas is not the rule, young patients must have an appropriate clinical and radiological follow up in the first six months with plain radiograms and MRI if needed.15 Especially if the excision is partial the infant must be re-examined for more than a year, in order to exclude rare malignant transformations to the remaining mesenchymal tissue.
Scoliosis of the thoracic vertebral column seems to be an inevitable late complication16. The grade of scoliosis and the decision to cure it, depends on the extension of the lesion and the number of the ribs affected. Scoliosis may present in young patients after just a plain thoracotomy for various interventions such as esophageal atresia repair, lobectomy or sequestration excision. Is obvious that the ablation of ribs and therefore the bereavement of the static support of the vertebral column leads to a grade of scoliosis. Confrontation depends on the grade of columnar deviation, the age and the provoked clinical problems.17,18 Orthopedic surgeons, neurologists and pneumonologists must collaborate to decide the need of surgical repair.
The mesenchymal hamartoma of the thoracic cavity is a benign lesion that could be detected in the antenatal ultrasound study. The aim of the multislice low-dose CT scan during the neonatal period is critical to provide a 3D depiction of the tumor and to differentiate from other malignant lesions. Total surgical excision of the mass must be the aim of indispensable surgical operation; it should be in a tertiary hospital pediatric unit by multidisciplinary team approach. The prognosis is optimal despite the aggressive surgical intervention during the neonatal period and the follow-up spinal intervention that may be required. Paradox respiratory movement is transitory and the main problem to confront is the scoliosis of the thoracic vertebral column. Close follow up is mandatory.
None.
Author declares there are no conflicts of interest.
None.

©2016 Ypsilantis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.