Journal of
eISSN: 2373-4426


Research Article Volume 11 Issue 4
1Department of Nursing, College of Health Science and Medicine, Dilla University, Ethiopia
2Department of Nursing, College of Health Science and Medicine, Dilla University, Ethiopia
3School of Nursing, College of Health Science, Debre Berhan University, Ethiopia
Correspondence: Migbar Sibhat, Department of Nursing, College of Health Science and Medicine, Dilla University, Dilla, Ethiopia
Received: November 18, 2021 | Published: December 29, 2021
Citation: Mekonnen MS, Nigussie TM, W/tsadik DA. Time to switching to second-line antiretroviral therapy and its predictors among HIV/AIDS infected children, Northern Ethiopia, 2020. J Pediatr Neonatal Care. 2021;11(4):75-84. DOI: 10.15406/jpnc.2021.11.00447
Background: With expanding access to pediatric antiretroviral therapy, a growing amount of patients in the developing world has switched to second-line therapy, and some requiring third-line medications. A delay in switch increases mortality and risk of developing opportunistic infections. There remain limited and often conflicting estimates on the use of second-line ART in children. Thus, this study intended to determine the incidence and predictors of switching to second-line antiretroviral therapy among children.
Methods: Retrospective follow up study was conducted. Single population proportion formula was used to estimate the sample size and all charts were taken for review. Data were collected by extraction tool; entered using Epi-data manager; cleaned and analyzed by Stata V-14. Kaplan-Meier curve, log-rank test, life table, and crude hazard ratios were used for data description and adjusted hazard ratios and p-value for analysis by Cox proportional hazard regression. Any variable at P≤0.25 in the bi-variable analysis was taken to multivariate analysis and significance was declared at P≤0.05. Data were presented using texts, tables, and figures.
Results: An overall 424 charts were incorporated for analysis. The total person-time observation was 11686.1 child-months with the incidence switch rate of 5.6 (95% CI 4.36-7.09) per 1000 child-months of observation. Being orphaned [AHR=2.36; 95%CI: 1.10-5.07], suboptimal ART adherence [AHR= 2.10; 95% CI: 1.12-3.92], drug toxicity [AHR= 7.05; 95% CI: 3.61-13.75], advanced recent WHO stage [AHR=2.75; 95%CI: 1.05-7.15], and initiating ART with TB co-infection [AHR=3.08; 95%CI: 1.26-7.51] were significantly associated with switch to second-line ART regimen. Moreover, long duration of ART follow up [AHR=0.75; 95% CI: 0.71-0.81] was found to be protective against switching.
Conclusion and recommendation: A remarkable delay in switching to second-line ART drugs was observed. Having sub-optimal adherence, baseline TB infection, advanced WHO stage on follow-up, ART toxicity, being an orphan, and duration of follow up were independent predictors of switching. Hence, it is better to give priority for strengthening the focused evaluation of tuberculosis co-infection and treatment failure with continuous adherence monitoring. Further research is also needed to evaluate the effect of drug resistance.
Keywords: Switching, ART, children, Ethiopia, predictors
The short-term effectiveness of ART among children is undisputed.1-5 A growing amount of patients in the developing world has switched to second-line therapy and some requiring third line medications.6-8 Highly Active Anti-Retroviral Therapy (HAART) refers drugs that serve to increase the life expectancy of children infected with HIV. It may be impossible to provide an effective and sustainable therapeutic regimen due to a lack of active combination agents against extensively drug-resistant viruses or because a patient is unable to adhere to or tolerate ART.9
Switching to second-line ART was defined as changing ≥2 new drugs including a class-switch from PI to NNRTI or vice versa irrespective of reasons, or changing two drugs with documented treatment failure, or change of both NRTIs and change from RTV to LPV/r with reason documented as treatment failure.10-12
There remain limited and often conflicting estimates on the use of second-line ART in children, with wide variations in both clinical trials and observational cohorts, ranging from 2%–35% switching at 2-5 years after ART initiation worldwide.3,4,13-15 Nonetheless, concerns have been raised that patients may be experiencing long periods with virologic failure.11,16 Many individuals who failed for first-line ART in sub-Saharan Africa never initiate second-line ART or do so after significant delay.15,17
Delays in scouting up treatment failure and shifting to second-line combination ART are frequently noted among HIV-infected children in LMIC.18,19 A delay in switch increases mortality and risk of developing opportunistic infections.17,20 Prolonged treatment with a failed regimen could result in 46% raised chance of failure to second-line therapy,20,21 increased drug toxicity,22 and increased drug resistance23 which may end up with exhaustion to available treatment and drive up program costs.15,22,24 This implies, in settings without access to resistance tests, more evidence is needed to refine the monitoring strategy for detecting treatment failure and switching regimens.15,25
Ethiopia is one of the high HIV-burden countries and does not have appropriate ART drug formulations for children beyond 2nd line.11 A solitary study in Ethiopia at Black Lion hospital regarding this area of study, which determined only the incidence of the switch to second-line ART, reported that among those children who failed to respond for the first-line regimen 14.4% were switched to second-line ART with a mean delay of 24 months.20
Albeit, this significant delay in switching and scarcity of data related to this topic, there are no studies conducted to evaluate factors that predict switching to second-line ART regimens among children throughout the country. Hence, this study designed to assess the time from ART initiation to second-line switch and to identify factors that predict a switch.
An institution-based retrospective follow-up study was employed at general hospitals in Mekelle and Southern zones of Tigray region, Ethiopia from December 2018- June 2019 by reviewing charts of children under 18 years of age who started to receive ART from January 2014 to December 2018. The total population of Mekelle city was 423,172; of which 214,141 were males and 208,931 were females. Whereas, 756,515 people live in the Southern zone of the Tigray region; of those 371,692 were males and 384,823 were females.26 There are five general governmental hospitals in the two zones (Mekelle General Hospital, Quiha General Hospital, Alamata General Hospital, Maichew General Hospital, and Korem General Hospital), all of which provide ART care services and therefore were eligible for selection.
Study population
All HIV/AIDS infected children before 18th birthday who were taking first-line ART at selected public general hospitals in Mekelle and Southern zones of Tigray region. Those charts of children with incomplete documentation (e.g initial ART regimen and substituted regimen) and no visit after ART initiation were excluded.
Sample size determination and sampling procedure
The sample size was determined using single population proportion formula considering the following assumptions: Zα/2 =1.96 (at 95% confidence level), 47.9% proportion of switch (p) and 52.1% proportion of survivors (1-p) from a study conducted at Black Lion hospital27 which brought the largest sample size, and 5% degree of freedom that finally yields 383.4. Thus, the total sample size required for the study was 384. Afterward, the hospitals were selected using a cluster sampling technique considering each hospital as a cluster constructing five clusters. Among those clusters, lottery method was used to select the three hospitals (Mekelle general hospital, Alamata general hospital, and Lemlem Karl hospital), and five years ART data were reviewed from charts of all children receiving first-line ART at the selected hospitals (clusters).
Operational definitions and measurements
Event /Switch was considered when (i) commencement of ≥2 new drugs including a class-switch from PI to NNRTI or vice versa irrespective of the cause, (ii) addition of new drug class, or (iii) change of ≥1 NRTIs or change from RTV to LPV/r with reason documented as treatment failure. Treatment failure refers to either clinical (new or repeated event indicative of WHO stage 3 or 4), immunologic (CD4 <200cells/L for older children and <100 cells/L for under 5), and/or virologic criteria (VL>1000copies/ml) after 6 months of effective ART treatment such that at least one criterion should be fulfilled.12 Censored were those who did not switch to the second-line regimen during follow-up including lost, transfer outs, died, exceed 18th birthday during follow up, and on first-line at the end of follow-up. Children were at risk from ART start until the earliest of switch or censor. The time to occurrence of an event or censored cases was measured in months. A patient was considered a defaulter if no follow-up visit for ≥ 3 months and adherence was measured based on the 2017 national ART score cut-offs.11,12
Data collection instruments and procedure
A data extraction checklist was developed from the national comprehensive HIV treatment guideline,11 ART registration booklet, ART monitoring multi-chart, and reviewing related articles. The extraction tool was comprised of socio-demographic characteristics, clinical and laboratory-related factors, treatment-related and other factors. The lists of participants were taken from the ART data clerk and unique ART numbers were used to find charts from the hospital card room. Four data collectors (BSc nurses) and one supervisor (ART trained MSc student) were recruited and the data collection was accomplished from April 1-26 /2019.
Data processing, analysis, interpretation, and presentation
At the end of data collection, data were entered into Epi-data manager version 4.4.2.1 and exported to STATA version 14 for cleaning, edition, coding, and analysis. The nature of data such as normality and presence of outliers as well as the levels of missing values were determined before description and analysis of data. Then the data were described using relative and expected frequency, percent, and median. Life-table was used to estimate the cumulative probabilities of switching at different time intervals. Kaplan Meier's curve was considered to estimate median switching time during the follow-up period and log-rank tests to compare survival curves for the presence of difference in the incidence of second-line switch among the groups.
The bi-variable analysis was carried out to identify possible associations between switching and each covariate. Those variables having P<=0.25 were included in the multivariate analysis, to identify independent predictors of a switch. Multi co-linearity was checked using the variance inflation factor (mean vif=1.33). Furthermore, proportional hazard assumptions were checked using the global test (p> χ2 =0.26). Harrell’s C was also computed (C=0.9935), which indicates that this study can correctly order survival times for pairs of children 99.4% of the time based on observations of fitted variables in the model. The Cox regression model for its fitness to the data was checked using the Cox-Snell residuals. The Log-likelihood test was also significant at prob> chi2 <0.001. Generally, we could conclude that the final model fits the data successfully. In the multivariate analysis, any statistical test was considered significant at P≤0.05. Then, the association between second-line switch and independent variables was declared by using an adjusted hazard ratio with 95% CI. Finally, texts, tables, and graphs used to present the results.
Data quality assurance
To assure the quality of data, data collectors and supervisors received one-day training about how and what information they should collect from the child charts. The checklist was pretested on 5% (20) of randomly selected charts at Ayder comprehensive specialized hospital, which was not included in the actual study. The principal investigator and supervisor audited the collected data daily. Whenever there appear incompleteness and uncertainty of recording, the filled information was crosschecked with source data soon. Individual records with incomplete data during data collection were excluded.
Ethical consideration
Ethical approval was received from the institutional review board (IRB) of Mekelle University, college of health sciences. Then, an official letter of cooperation was written from the school of nursing and permission for data collection was obtained from selected hospitals on behalf of patients and ART clinics of each hospital since the study was conducted through a review of medical records. Finally, data were collected from the charts of HIV/AIDS infected children. For certainty of anonymity and confidentiality of data, data were coded and reported as combined.
From the beginning of 2014 to the end of 2018, a total of 502 (198 cases from Alamata, 165 from Mekelle and 139 from Lemlem Karl hospitals) children below 18 years of age were started ART. Of those, 78 charts (69 records because of missing data and nine with no follow-up after ART initiation) were excluded from the analysis. Finally, four hundred twenty-four (N=424) children aged less than 18 years who fulfilled the eligibility criteria were incorporated for analysis from three general hospitals of Mekelle and Southern zone of Tigray region.
Socio-demographic characteristics
Children were followed for a minimum of 6 months and a maximum of 60 months with a median follow-up of 24.4 months from the beginning of 2014 to the end of 2018. The study finding showed that two hundred eleven (49.8%) children were males, of which 16.6% fulfilled the switch criteria while this was true among 14.4% of female participants. The median age of children at ART initiation was 9 years with a minimum of 6 and a maximum of 214 months at the start. A significant proportion (23.3%) of children aged 5-10 years met at least one of the switch criteria followed by >10 years of age (14.6%). The study result also revealed that 154 (36.3%) children were orphaned who lost either one or both parents; of which 34 (22.1%) eligible for second-line ART regimens (Table 1).
|
|
ART Outcome |
|||||
|
Independent variables |
Categories |
Switched |
Not switch |
Total |
||
|
Observed (%) |
Expected |
Observed (%) |
Expected |
Count (%) |
||
|
Age at ART initiation |
<5 yrs |
6 (5.5) |
16.9 |
104 (94.5) |
93.1 |
110 (25.9) |
|
5-10 |
35 (23.3) |
23 |
115 (76.7) |
127 |
150 (35.4) |
|
|
>10 |
24 (14.6) |
25.1 |
140 (85.4) |
138.9 |
164 (38.7) |
|
|
Sex |
Female |
30 (14.1) |
32.7 |
183 (85.9) |
180.3 |
213 (50.2) |
|
Male |
35 (16.6) |
32.3 |
176 (83.4) |
178.7 |
211 (49.8) |
|
|
Parent Status |
Both alive |
31 (11.5) |
41.4 |
239 (88.5) |
228.6 |
270 (63.7) |
|
Either Died |
19 (19.4) |
15 |
79 (80.6) |
83 |
98 (23.1) |
|
|
Both Died |
15 (26.8) |
8.6 |
41 (73.2) |
47.4 |
56 (13.2) |
|
Table 1 Distribution of socio-demographic characteristics among HIV/AIDS infected children in public general hospitals, Northern Ethiopia, 2020 (N=424)
Abbreviation: ART, antiretroviral therapy
Clinical and laboratory-related characteristics
The study finding notified that 50% of the participants had less than 475cells/mm3 at the initiation. About 81% and 58.5% of children initiated ART with less than 3rd percentile HFA and WFA respectively. On the other way, 111 (26.2%) children who started ART with advanced WHO clinical stage 25 (22.5%) were identified to be switched to second-line ART regimens whereas 20.4% of those started with CD4 count less than 200cells/mm3 fulfilled the switch criteria. Meanwhile, 201 (47.4%) children had no access to a viral load investigation (Table 2).
|
|
ART Outcome |
|||||
|
Independent variables |
Category |
Switched |
Not switched |
Total (%) |
||
|
Observed (%) |
Expected |
Observed (%) |
Expected |
|||
|
WFA at baseline |
< 3rd |
39 (15.7) |
38 |
209 (84.3) |
210 |
248 (58.5) |
|
3rd - 97th |
26 (15.2) |
26.2 |
145 (84.8) |
144.8 |
171 (40.3) |
|
|
> 97th |
0 |
0.8 |
5 (100) |
4.2 |
5 (1.2) |
|
|
HFA at baseline |
< 3rd |
54 (15.7) |
52.6 |
289 (84.3) |
290.4 |
343 (80.9) |
|
3rd - 97th |
6 (11.1) |
8.3 |
48 (88.9) |
45.7 |
54 (12.7) |
|
|
> 97th |
5 (18.5) |
4.1 |
22 (81.5) |
22.9 |
27 (6.4) |
|
|
WHO stage at ART start |
Early |
40 (12.8) |
48 |
273 (87.2) |
265 |
313 (73.8) |
|
Advanced |
25 (22.5) |
17 |
86 (77.5) |
94 |
111 (26.2) |
|
|
WHO stage at last visit |
Early |
56 (14.2) |
60.4 |
338 (85.8) |
333.6 |
394 (92.9) |
|
Advanced |
9 (30) |
5 |
21 (70) |
25.4 |
30 (7.1) |
|
|
CD4 count at baseline |
≤200 |
10 (20.4) |
7.5 |
39 (79.6) |
41.5 |
49 (11.6) |
|
>200 |
42 (16.3) |
39.6 |
216 (83.7) |
218.4 |
258 (60.8) |
|
|
Unknown |
13 (11.1) |
17.9 |
104 (88.9) |
99.1 |
117 (27.6) |
|
|
The most recent CD4 count |
≤200 |
15 (53.6) |
4.3 |
13 (46.4) |
23.7 |
28 (6.6) |
|
>200 |
26 (12.9) |
30.8 |
175 (87.1) |
170.2 |
201 (47.4) |
|
|
Unknown |
24 (12.3) |
29.9 |
171 (87.7) |
165.1 |
195 (46) |
|
|
Access to viral load |
No |
14 (7) |
30.8 |
187 (93) |
170.2 |
201 (47.4) |
|
Yes |
51 (22.9) |
34.2 |
172 (77.1) |
188.8 |
223 (52.6) |
|
|
VL at initiation |
<1000 |
6 (13.6) |
6.7 |
38 (86.4) |
37.3 |
44 (10.4) |
|
≥1000 |
7 (35) |
3.1 |
13 (65) |
16.9 |
20 (4.7) |
|
|
Unknown |
52 (14.4) |
55.2 |
308 (85.6) |
304.8 |
360 (84.9) |
|
|
Recent VL |
<1000 |
23 (14.2) |
24.8 |
139 (85.8) |
137.2 |
162 (38.2) |
|
≥1000 |
28 (47.5) |
9 |
31 (52.5) |
50 |
59 (13.9) |
|
|
Unknown |
14 (6.9) |
31.1 |
189 (93.1) |
171.9 |
203 (47.9) |
|
Table 2 Clinical and laboratory-related characteristics of HIV/AIDS infected children in public general hospitals, Northern Ethiopia, 2020 (N=424)
Abbreviations: ART, antiretroviral therapy; WFA, weight for age; HFA, height for age; WHO, world health organization; VL, viral load
Considerably, study participants acquired opportunistic infections at baseline as well as after initiation of ART were 38.9% and 25.2% respectively. Moreover, 20.6% and 27.1% of those children having OI at baseline and after initiation respectively met the switch criteria whereas only 12% of those started ART without OIs and 11.4% of those developed OIs after initiation satisfied the criteria to switch to second-line regimens (Table 3).
|
Covariates |
Category |
ART Outcome |
|
||||
|
Switched |
|
Not switched |
Total (%) |
||||
|
Observed (%) |
Expected |
Observed (%) |
Expected |
||||
|
Opportunistic infections at baseline
|
No |
31 (12) |
39.7 |
228 (88) |
219.3 |
259 (61.1) |
|
|
Yes |
Anemia |
1 (6.7) |
2.3 |
14 (93.3) |
12.7 |
15 (3.5) |
|
|
Diarrhea |
13 (20.3) |
9.8 |
51 (79.7) |
54.2 |
64 (15.1) |
||
|
SAM |
6 (17.1) |
5.4 |
29 (82.9) |
29.6 |
35 (8.3) |
||
|
TB |
14 (27.5) |
7.8 |
37 (72.5) |
43.2 |
51 (12) |
||
|
Pneumonia |
19 (25) |
11.7 |
57 (75) |
64.3 |
76 (17.9) |
||
|
URTI |
6 (24) |
3.8 |
19 (76) |
21.2 |
25 (5.9) |
||
|
UTI |
3 (75) |
0.6 |
1 (25) |
3.4 |
4 (0.9) |
||
|
Candidiasis |
1 (33.3) |
0.5 |
2 (66.7) |
2.5 |
3 (0.7) |
||
|
Oral thrush |
2 (20) |
1.5 |
8 (80) |
8.5 |
10 (2.4) |
||
|
Meningitis |
1 (20) |
0.8 |
4 (80) |
4.2 |
5 (1.2) |
||
|
Total |
34 (20.6) |
25.3 |
131 (79.4) |
139.7 |
165 (38.9) |
||
|
Opportunistic infections after ART initiation
|
No |
36 (11.4) |
48.6 |
281 (88.6) |
268.4 |
317 (74.8) |
|
|
Yes |
Anemia |
9 (34.6) |
4 |
17 (65.4) |
22 |
26 (6.1) |
|
|
Diarrhea |
6 (21.4) |
5 |
22 (78.6) |
23.7 |
28 (6.6) |
||
|
SAM |
6 (22.2) |
5 |
21 (77.8) |
22.9 |
27 (6.4) |
||
|
TB |
10 (31.2) |
5 |
22 (68.8) |
27.1 |
32 (7.5) |
||
|
Pneumonia |
13 (31.7) |
6.3 |
28 (68.3) |
34.7 |
41 (9.7) |
||
|
URTI |
6 (31.6) |
2.9 |
13 (68.4) |
16.1 |
19 (4.5) |
||
|
UTI |
0 |
0.6 |
4 (100) |
3.4 |
4 (0.9) |
||
|
Candidiasis |
2 (22.2) |
1.4 |
7 (77.8) |
7.6 |
9 (2.1) |
||
|
Oral thrush |
1 (50) |
0.3 |
1 (50) |
1.7 |
2 (0.5) |
||
|
Meningitis |
3 (42.9) |
1.1 |
4 (57.1) |
5.9 |
7 (1.7) |
||
|
Total |
29 (27.1) |
16.4 |
78 (72.9) |
90.6 |
107 (25.2) |
||
Table 3 Distribution of Opportunistic Infections among HIV/AIDS infected children in public general hospitals, Northern Ethiopia, 2020 (N=424)
Abbreviations: ART, antiretroviral therapy; SAM, severe acute malnutrition; TB, tuberculosis; URTI, upper respiratory tract infection; UTI, urinary tract infection
Treatment-related and other factors
Three hundred twenty-nine (80.9%) participants started with NNRTIs based ART regimen with NVP-based dominating with 200 (47.2%) whereas 46 (10.8%) and 35 (8.3%) children started ABC-based and boosted PI-based regimens respectively. Sixty-four (15.1%) children had previous exposure to ART for either PMTCT or PEP and 31 (7.3%) did not take any OI prophylaxis. One hundred twenty (28.3%) children have not disclosed their serostatus on ART start; 117 (27.6%) had suboptimal adherence as well as 133 (31.5%) and 101 (23.8%) developed adverse effects and substituted their initial first-line regimen during follow up respectively (Table 4).
|
|
|
What was the last outcome |
||||||
|
Independent variables |
Category |
Switched |
Not switched |
Total (%) |
||||
|
Observed (%) |
Expected |
Observed (%) |
Expected |
|||||
|
Previous ART exposure |
No |
61 (16.9) |
55.2 |
299 (83.1) |
304.8 |
360 (84.9) |
||
|
Yes |
4 (6.2) |
9.8 |
60 (93.8) |
54.2 |
64 (15.1) |
|||
|
OI prophylaxis |
No |
1(3.2) |
4.8 |
30 (96.8) |
26.2 |
31 (7.3) |
||
|
Yes |
64 (16.3) |
60.2 |
329 (83.7) |
332.8 |
393 (92.7) |
|||
|
Disclosure status |
No |
18 (15) |
18.4 |
102 (85) |
101.6 |
120 (28.3) |
||
|
Yes |
47 (15.5) |
46.6 |
257 (84.5) |
257.4 |
304 (71.7) |
|||
|
Adherence to ART |
Sub-optimal |
Poor |
16 (25.8) |
9.5 |
46 (74.2) |
52.5 |
62 (14.6) |
|
|
Fair |
12 (21.8) |
8.4 |
43 (78.2) |
46.6 |
55(13) |
|||
|
Optimal |
Good |
37 (12.1) |
46.9 |
270 (87.9) |
259.1 |
307 (72.4) |
||
|
Baseline ART regimen |
NVP-based |
37 (18.5) |
30.7 |
163 (81.5) |
169.3 |
200 (47.2) |
||
|
ABC-based |
5 (10.9) |
7.1 |
41(89.1) |
38.9 |
46 (10.8) |
|||
|
EFV-based |
19 (13.3) |
21.9 |
124 (86.7) |
121.1 |
143 (33.7) |
|||
|
LPV/r-based |
4 (11.4) |
5.4 |
31(88.6) |
29.6 |
35 (8.3) |
|||
|
ART drug Toxicity |
No |
13 (4.5) |
44.6 |
278 (95.5) |
246.4 |
291 (68.6) |
||
|
Yes |
52 (39.1) |
20.4 |
81(60.9) |
112.6 |
133 (31.4) |
|||
|
ART drug substitution |
No |
0 |
49.5 |
323 (100) |
273.5 |
323 (76.2) |
||
|
|
PI to NNRTI |
44 (100) |
6.7 |
0 |
37.3 |
44 (10.4) |
||
|
<2 NRTI |
11 (57.9) |
2.9 |
8 (42.1) |
16.1 |
19 (4.5) |
|||
|
New class added |
10 (100) |
1.5 |
0 |
8.5 |
10 (2.3) |
|||
|
within NNRTI |
0 |
4.3 |
28 (100) |
23.7 |
28 (6.6) |
|||
|
Treatment failure |
Yes |
42 (43.8) |
14.7 |
54 (56.2) |
81.3 |
96 (22.6) |
||
|
No |
23 (7) |
50.3 |
305 (93) |
277.7 |
328 (77.4) |
|||
Table 4 Distribution of treatment-related and other factors among HIV/AIDS infected children in public general hospitals, Northern Ethiopia, 2020 (N=424)
Abbreviations: ART, antiretroviral therapy; OI, opportunistic infections; PI, protease inhibitors; NNRTIs, nonnucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; NVP, nevirapine; ABC, abacavir; EFZ, Efavirenz; LPV/r, lopinavir/ritonavir
Among the total 424 children in the study, 65 (15.33%) satisfied at least one switch criterion of which only 31 (47.7%) children switched to second-line ART regimens while the rest 52.3% of those fulfilled the criteria remained on first-line. The rest 359 (84.67%) were censored with seven (1.7%) children died during follow up, 61 (14.4%) transferred out to other facilities, and 35 (8.2%) lost as defaulters during the follow-up period. In addition, two-third (64.6) of those considered, as switch were attributable to first-line treatment failure.
Comparison of survival status using kaplan meier
The Kaplan Meier switch curve increased stepwise as the follow-up time increased and it crosses the survival function at a survival probability of 0.5 (Figure 1).
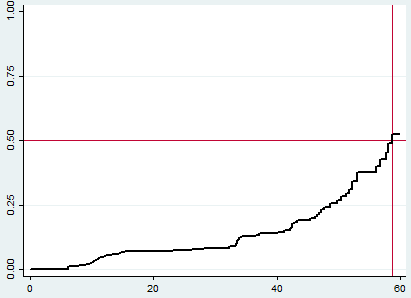
Figure 1 Overall Kaplan Meier failure estimate of HIV/AIDS infected children in public general hospitals, Northern Ethiopia, 2020 (N=424). The Y-axis represents the probability of second-line ART switch whereas the X-axis indicates the follow-up time in months. The red vertical and horizontal lines were reference lines added to ease graph interpretation (median time to switch estimation).
Survival function and an incidence density rate of a switch to second-line ART among children receiving first-line ART
The total person-time observation was 11686.1 child-months with the incidence switch rate of 5.6 (95% CI 4.36-7.09) per 1000 child-months of observation or 6.7 per 100 child-years of observation. The median survival time was found to be 58.7months. The cumulative probabilities of switch at 12, 24, 36, 48 and 60 months were 0.053, 0.08, 0.13, 0.24 and 0.52 respectively.
Predictors of switching to second-line ART
After the description of the data, the bi-variable analysis was conducted using the Cox proportional hazard regression model to determine the variables that should be fitted to the final model for multivariate analysis. Some variables such as WFA, HFA, recent CD4 count, baseline VL, drug substitution, and OI prophylaxis were left out of the final model since they have less than 20% predicted events per cell. Additionally, age at ART start and recent VL were further excluded for violation of proportionality assumptions, and pneumonia for multi-collinearity effect.
Afterward, in the final Cox proportional hazard model, Being orphaned, suboptimal ART adherence, drug toxicity, advanced recent WHO stage, initiating with TB co-infection at baseline and duration of follow-up were found to be independent predictors of switching to second-line ART regimen (Table 5).
|
|
Last outcome |
P>|z| |
Crude HR (95% CI) |
P>|z| |
Adjusted HR (95% CI) |
|||
|
Covariates |
Category |
Switched |
Not switched |
|||||
|
Parent status |
Both alive |
31 |
239 |
- |
- |
- |
- |
|
|
Either died |
19 |
79 |
0.026 |
1.92 (1.08 - 3.41) |
0.039* |
2.13 (1.04- 4.38)* |
||
|
Both died |
15 |
41 |
0.001 |
2.81 (1.52 - 5.22) |
0.027* |
2.36 (1.10- 5.07)* |
||
|
OI at baseline |
Yes |
34 |
131 |
0.012 |
1.87 (1.15 - 3.05) |
0.710 |
1.15 (0.56 - 2.37) |
|
|
No |
31 |
228 |
- |
- |
- |
- |
||
|
OI after ART initiation |
Yes |
29 |
78 |
<0.001 |
2.47 (1.51 -4.04) |
0.182 |
1.77 (0.77 - 4.09) |
|
|
No |
36 |
281 |
- |
- |
- |
- |
||
|
Adherence to ART drugs |
Optimal |
37 |
270 |
- |
- |
- |
- |
|
|
Sub-optimal |
28 |
89 |
<0.001 |
2.51 (1.53 - 4.11) |
0.021* |
2.10 (1.12 - 3.92)* |
||
|
ART exposure |
Yes |
4 |
60 |
- |
- |
- |
- |
|
|
No |
61 |
299 |
0.215 |
1.90 (0.69 - 5.23) |
0.139 |
0.43 (0.14 - 1.31) |
||
|
ART drug toxicity |
Yes |
52 |
81 |
<0.001 |
5.68 (3.08 - 10.49) |
<0.001* |
7.05 (3.61 - 13.75)* |
|
|
No |
13 |
278 |
- |
- |
- |
- |
||
|
Baseline WHO stage |
Early-stage |
40 |
273 |
- |
- |
- |
- |
|
|
Advanced stage |
25 |
86 |
0.011 |
1.91 (1.16 - 3.16) |
0.997 |
1.00 (0.50 - 2.00) |
||
|
Recent WHO stage |
Early-stage |
56 |
338 |
- |
- |
- |
- |
|
|
Advanced stage |
9 |
21 |
<0.001 |
4.20 (2.06 - 8.59) |
0.039* |
2.75 (1.05 - 7.15)* |
||
|
Baseline CD4 |
Failed |
10 |
39 |
0.127 |
1.72 (0.86 - 3.43) |
0.658 |
0.83 (0.36 - 1.92) |
|
|
|
Normal |
42 |
216 |
- |
- |
- |
- |
|
|
|
Unknown |
10 |
39 |
- |
- |
- |
- |
|
|
Anemia after ART start |
No |
56 |
342 |
- |
- |
- |
- |
|
|
Yes |
9 |
17 |
<0.001 |
4.51 (2.18 - 9.34) |
0.054 |
0.22 (0.05 - 1.02) |
||
|
Diarrhea after ART initiation |
No |
59 |
337 |
- |
- |
- |
- |
|
|
Yes |
6 |
22 |
0.121 |
1.95 (0.84 - 4.54) |
0.744 |
0.82 (0.26 - 2.62) |
||
|
Malnutrition at baseline |
No |
59 |
330 |
- |
- |
- |
- |
|
|
Yes |
6 |
29 |
0.168 |
1.81 (0.78 - 4.23) |
0.132 |
2.31 (0.78 - 6.89) |
||
|
Malnutrition after ART start |
No |
59 |
338 |
- |
- |
- |
- |
|
|
Yes |
6 |
21 |
0.039 |
2.45 (1.05 - 5.73) |
0.183 |
0.40 (0.11 - 1.53) |
||
|
TB at baseline |
No |
51 |
322 |
- |
- |
- |
- |
|
|
Yes |
14 |
37 |
<0.001 |
4.03 (2.21 - 7.38) |
0.013* |
3.08 (1.26 - 7.51)* |
||
|
TB after ART initiation |
No |
55 |
337 |
- |
- |
- |
- |
|
|
Yes |
10 |
22 |
0.093 |
1.79 (0.91 - 3.55) |
0.112 |
2.23 (0.83 - 5.98) |
||
|
Follow up duration |
65 |
359 |
<0.001 |
0.89 (0.88 - 0.91) |
<0.001* |
0.75 (0.71 - 0.81)* |
||
Table 5 Bi-variable and Multivariate analysis output for HIV/AIDS infected children in public general hospitals, Northern Ethiopia, 2020 (N=424)
Note: *significant at 5% level of significance
Abbreviations: OI, opportunistic infection; ART, anti retroviral therapy; WHO, world health organization; CD4, HIV helper cell count; TB, tuberculosis
Children who were either single or double orphaned had increased hazard of a switch to second-line regimens [AHR=2.13; 95%CI: 1.04-4.38] and [AHR=2.36; 95%CI: 1.10-5.07] respectively than those having both parents alive. In addition to this, children who diagnosed as advanced WHO stage at last visit had 2.75 times higher hazard than those who defined as in the early WHO stages [AHR=2.75; 95%CI: 1.05-7.15]. Likewise, the hazard of switch among children with sub-optimal adherence was 2.1 [AHR= 2.10; 95% CI: 1.12-3.92] times greater than those with good adherence.
Furthermore, children who had TB infection at ART start were observed to have three times higher hazard of a switch than their counterparts [AHR=3.08; 95%CI: 1.26-7.51]. The other factor that showed significant association was the duration of ART follow-up. Children who had been on ART for a long period were less likely to switch to second-line drugs. For each month increase in the duration of ART follow up, the hazard of developing treatment failure will decrease by 25% [AHR=0.75; 95% CI: 0.71-0.81]. Again, those children who developed drug adverse effects were also seven times at higher hazard of a switch than those with no drug toxicity during follow-up [AHR= 7.05; 95% CI: 3.61-13.75].
This study was aimed to determine the incidence and predictors of switching to second-line ART regimen among HIV/AIDS infected children. The median survival time was 58.7 months with an overall incidence switching rate of 5.6 (95% CI 4.36-7.09) per 1000 child-month-observations. Being orphaned, suboptimal ART adherence, drug toxicity, advanced recent WHO stage, baseline TB infection, and duration of follow-up were found to be independent predictors of the second-line switch.
In this study, the median survival time was 58.7 months which is longer than the findings of previous studies; 35 months from a global pooled estimate by CIPHER,28 and 30 months in Europe and Thailand.13 The possible explanation could be the advancements in the diagnostic and therapeutic measures recently including more frequent visits and increased access to viral load that enables early detection and monitoring of ART responses including adherence as well as the increase in access and variety of more potent drugs nowadays than in the past.11
The cumulative incidence of a switch at three years was 13% (95% CI 9.31- 17.69). This was in line with Europe and Thailand's study finding reported as 14%13 and higher than 3.1% reported in the global pooled analysis by CIPHER.28 On the other hand, the overall cumulative incidence of the switch at five years in this study was 52% (95% CI 39.61-66.34). This was higher than reports of previous studies; 31.6% in Asia-Pacific and African countries<29 and 21% in Europe and Thailand.13
Having tuberculosis at ART start was significantly associated with switching to second-line ART drugs [AHR=3.08; 95%CI: 1.26-7.51]. This could be rationalized as tuberculosis infection facilitates viral replication by activating the immune system, which then leads to viral load increment. Again, this will in turn accelerate rapid HIV/AIDS disease progression which cross-react with the ART drug action11 leading to increased replication of drug-resistant mutations and thus a higher chance of switching.
The other factor that showed significant association was ART drug toxicity. Children who developed drug adverse effects were seven times at higher hazard of a switch than those with no drug toxicity during follow-up [AHR= 7.05; 95% CI: 3.61-13.75]. This finding was supported by a previous study conducted in West African countries.30 This might be because intolerance to ART drugs is an important barrier to adherence leading to treatment discontinuation, risking viral rebound, and drug resistance.9,11 The major cause of drug discontinuation in the first 3-6 months after ART initiation is drug toxicity.
The study finding also notified that being orphan children was significantly associated with the switch to second-line regimens. From the beginning, children are dependent on their parents and require continual support due to their age and developmental stages. Parents play an undeniable role in the provision of effective pediatric ART service by encouraging children to take ART drugs timely, as prescribed, assist in case of sub-optimal adherence, attend follow-up based on appointments, and avoid the sense of loneliness. On the contrary, orphaned children will miss doses and follow-up visits, difficulty with adherence, and even may not fully understand healthcare instructions. Besides, poor palatability of drugs, frequent adverse effects, limited formulations, and frequent dosing requirements9,11 may lead to poor adherence and exacerbate the level of negligence.
This study revealed that children who diagnosed as advanced WHO stage at last visit were at higher hazard of a switch than those who defined as in the early WHO stages [AHR=2.75; 95%CI: 1.05-7.15]. Earlier pediatric studies did not examined the WHO stage at the last follow up visit. However, previous studies investigated the WHO stage at baseline and reported as an independent predictor of switching, which was not found statistically significant in the current study. The possible justification for the discrepancy could due to advancement in the management strategies and short follow-up visit recommendations in the recent ART guideline.11 Consequently, it will enable early detection, control, and management of adverse events, and opportunistic infections through strict and focused service provision for children who start ART with advanced WHO stage in terms of treatment regimen selection, diagnostic and monitoring workups, and repeated follow-up visits.
The current study also showed that those children having sub-optimal adherence for ART regimens were 2.1 times at higher hazard of switch compared to their counterparts [AHR= 2.10; 95% CI: 1.12-3.92]. This report was in agreement with the previous study result.30 The possible reason for this could be due to the role of a high level of sustained adherence to ART treatment outcomes. Optimal adherence is necessary to reduce the risk of ART drug resistance and decrease the chance of HIV transmission by suppressing viral replication and improving immunological and clinical outcomes. On the other hand, HIV/AIDS infected children and adolescents frequently come upon with poor adherence to ART drugs. Several reasons could be stated for this including limited choice of pediatric ART formulations, poor palatability of some drug preparations, the requirement of multiple pills with frequent dosing, as well as occurrence of potential adverse effects and drug interaction in pediatric regimens. Adherence could also be affected by the age and developmental stage of children since the pediatric age group needs support from others to take medication timely and also may face difficulties in swallowing tablets.9,11
A long duration of follow up was also reported as having a protective effect on a switch to second-line ART [AHR=0.75; 95% CI: 0.71-0.81]. This finding contradicts the results of a West African study.30 The difference could be, unlike the current study which incorporates all children started on first-line ART, the West African study considered children that experienced first-line ART failure and estimated switch to second-line among only those who failed for first-line ART drugs. We can also put justifications for our results. Children who had been on ART for a prolonged period will have improved adherence and adaptation. This is because the level of adherence in children increases with time, and the need for adaptation to daily ART drug intake in the early ART follow-up periods may hamper the patient's response to ART drugs resulting in inadequate viral suppression and thus the emergence of resistant mutations.9 This may also be related to the increased chance of occurrence of different rapid effects such as IRIS within the early months of ART initiation.
The overall cumulative incidence of the second-line switch was higher than in previous studies. A remarkable delay in switching to second-line ART drugs was observed. Furthermore, children who had ART drug toxicity, TB at ART initiation, advanced WHO clinical stage after ART initiation, non-adherence to ART regimen, those who were orphaned, and on ART for a short period were at higher hazard of switching.
The authors would like to appreciate data collectors, supervisors, hospital staff, and administrators for their unreserved efforts and commitment. Besides, the authors acknowledged Dilla University for funding this study and Mekelle University to offer this chance.
The authors declare no conflicts of interest.
Ethical clearance and ethical approval were obtained from the Intuitional Review Board (IRB) of Mekelle University. The IRB waived such that the research could be done by record review without contacting patients since the study was retrospective. A cooperation letter was obtained from Mekelle University, School of Nursing. Permission letters were obtained from each hospital administration and respective hospital ART coordinators. All information was kept confidential and no individual identifiers were collected.
MSM conceived and designed the study, participated in the collection and interpretation of data. MSM, TMN & DAW act in proposal development, edited the proposal, performed the statistical analysis as well as drafted and critically revised the manuscript. MSM edited the manuscript and formatted it for publication. All authors read and approved the final manuscript and agreed to be accountable for all aspects of the work.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. However, Dilla University covered the financial backing of this research. The funder had no role in study design, data collection, analysis, preparation of the manuscript, and decision to publish.
Extra data that support the findings of this study are available from the corresponding author upon reasonable request and can be shared upon legal request via ‘migbaresenay150@gmail.com’ OR ‘bayayibignabez@gmail.com’.
Not applicable.

©2021 Mekonnen, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.