Journal of
eISSN: 2373-4426


Case Report Volume 9 Issue 6
Department of Pediatrics, East Tennessee State University, USA
Correspondence: Des Bharti, Professor of pediatrics, Quillen College of Medicine, East Tennessee State University, Johnson City, Tennessee 37614, USA
Received: October 23, 2019 | Published: November 1, 2019
Citation: Bharti D, Patel I. Subcutaneous fat necrosis in an extremely low birth weight infant. J Pediatr Neonatal Care. 2019;9(5):147-150. DOI: 10.15406/jpnc.2019.09.00397
We are reporting an infant delivered at 23 weeks of gestation who presented with subcutaneous fat necrosis (SCFN).
Keywords: Subcutaneous fat necrosis, SCFN, extremely premature infant
Subcutaneous fat necrosis (SCFN) is a relatively uncommon condition, generally reported in term or post-term infants.1 The condition is usually benign but potentially can lead to complications like growth delay, dyslipidemia, renal failure and subcutaneous atrophy. 25-50% of infants have hypercalcemia, 77% have skin lesions.2,3 76% have resolution of hypercalcemia within 4 weeks of age. Hypercalcemia is generally noted in infants with higher birth weights. The mean age for development of skin lesions is three to four days after birth. A significant number of infants with subcutaneous fat necrosis have diffused lesions over the body. SCFN could be potentially associated with forceps delivery, maternal diabetes, maternal hypertension, and history of maternal smoking.4 Late subcutaneous atrophy has been reported in infants after resolution of subcutaneous fat necrosis. Subcutaneous fat necrosis has also been reported in infants requiring body cooling for hypoxic ischemic encephalopathy.5,6 Other potential associated complications are thrombocytopenia, hyperglycemia, hypertriglyceridemia and metastatic calcifications in kidneys or myocardium.7,8
A 550 g, 23 weeks and 6 day gestation, female infant was born by precipitous vaginal delivery with Apgars scores of 4 at one, 7 at five and 7 at ten minutes. The mother presented with vaginal bleeding with clots and had a rapid dilatation of the cervix. She was 35 year old, G1 para zero, white female. The pregnancy was complicated by gestational diabetes. She was on insulin therapy. She had chronic hypertension with history of tachycardia requiring metoprolol daily. She received one dose of betamethasone, IV magnesium sulfate and IV penicillin G prior to delivery. The amniotic fluid was clear. The prenatal labs were unremarkable, maternal blood type is O-positive, and her group B strep status was unknown.
The infant was noted to be in severe respiratory distress soon after birth. She was given positive pressure ventilation via face mask followed by intubation. A dose of surfactant was given in delivery room. On admission to neonatal ICU, the infant was electively connected to high-frequency oscillator with mean airway pressure of 9, amplitude of 22, and frequency of 15 hertz. Initial chest x-ray was consistent with severe respiratory distress syndrome manifesting as reticular granular pattern bilaterally. Umbilical artery and venous catheters were placed. The initial blood gas showed a pH of 7.33, pCO2 43, PO2 56, base excess -3.2. The infant was started on amino acid dextrose solution to provide fluids at 100 mL per kg for first 24 hours. The infant had a length of 32 cm, and head circumference of 19.5 cm. Initial white blood cell count was 11,000/uL, hematocrit 41.1%, platelet count 292,000/uL. The infant was not started on antibiotics. A blood culture was sent and was subsequently reported negative.
At about 24 hours of age, the infant was noted to have a mean arterial blood pressure less than 25 mm of Hg with blanching of the skin in the peri-umbilical region, and purplish plaque over the left flank with intact overlying skin (Figure 1). Initially, these skin lesions were attributed to use of skin disinfectant, chloaraprep, containing chlorhexidine and isopropyl alcohol. The disinfectant was applied to the umbilical area prior to umbilical catheterization and was partially diluted with distilled water. Because of persistent hypotension, the infant was started on dopamine at five microgram/kilogram per minute and hydrocortisone was added at one milligram per kilogram per dose every eight hours. At the same time, the infant had clinical worsening of the respiratory status with metabolic and respiratory acidosis requiring high-frequency jet ventilation. An additional dose of surfactant was given. The echocardiogram showed small PDA, normal cardiac function, and mild tricuspid regurgitation. Initial serum calcium level was 9.1 mg/dL that increased to 11.1 mg/dL on day two of life. At this time, the infant was not getting any supplemental calcium in the IV fluids. The serum phosphorus level was 2.5 mg/dL. The infant continued to have high normal serum calcium level ranging from 10.1 to 11.1 for first week of life. The urine output and renal function stayed in normal range. Serum creatinine ranged from 0.56 to 0.73 mg/dL. Intravenous supplementation of calcium in the total parental nutrition was added on day four of life. Serum calcitonin level on day two of life was 32.6 picograms/mL and serum 1,25 hydroxy vitamin D was reported at 47.5 picograms/mL. The feeding protocol was started on day two of life. Renal sonogram did not show any evidence of nephrocalcinosis.
There was gradual improvement in skin perfusion and mean arterial blood pressure improved to above 30 mm of Hg. Dopamine was discontinued within 24 hours and infant was weaned off IV hydrocortisone within 48 hours. On day three of life, placental pathology indicated findings consistent with chorioamnionitis. The infant was given one-week course of ampicillin and gentamicin. On day four of life, the infant had serum sodium of 156meq/dL, requiring total IV fluids of about 180 mL/Kg. By day six of life, serum sodium was in normal range and the total fluids were decreased to 130 mL per kilogram. On day three of life, the infant had hematocrit of 22% associated with skin pallor. Infant was given a transfusion of packed red blood cells at 15 mL/kg. Next CBC on the same day showed a hematocrit 26% and the infant was given an additional dose of packed red blood cells at 15mL/kg. On day four of life, the infant had platelet count of 82,000/ml and she was given one platelet transfusion of 10 mL/kg. Head sonogram on day three of life was suggestive of bilateral grade three intraventricular hemorrhage and repeat study at week one indicated enlarging grade three intraventricular hemorrhage with small left frontal parenchymal hemorrhage.
(Parental permission was obtained for the pictures of the skin lesions)
Subcutaneous fat necrosis is a relatively uncommon occurrence in extremely premature infants. It is primarily a disorder affecting full-term or post-term infants within 1st few weeks of life. It is a benign and self-recovering condition. There are no reported cases of subcutaneous fat necrosis in extremely premature infant. It is very important to differentiate subcutaneous fat necrosis from several other clinical situations. The skin of an infant is the most important organ for maintaining body temperature regulation, to secrete moisture, to maintain the barrier from the atmosphere and external environment. It is the first-line of defense for basic immune system and it protects the body from potentially harmful substances and bacteria. The skin of extremely preterm infant is porous, relatively thin, and has a limited amount of subcutaneous fat. The skin breakdown and scattered bruises are not uncommon in micro premature infants that can develop with minor trauma or mere touch to skin. Premature infants under 28 weeks of gestation lack coverage with vernix caseosa and have an underdeveloped stratum corneum barrier. They experience high water loss, electrolyte imbalance, thermal instability and increased risk of infections. The stratum corneum in a term infant is multiple layer thick and has sufficient fat but in an infant under 24 weeks of life, the stratum corneum is barely 3-5 layer thick.9
SCFN is an uncommon lobular panniculitis, presenting with single or multiple areas of erythema, purplish plaques and nodules. The affected area of skin could potentially get calcified, get hemorrhagic or have superficial skin breakdown. These lesions are at higher risk of local and systemic bacterial sepsis. Micro premature infants born by cesarean section and vaginal delivery are at higher risk of bruises, overall skin breakdown, and risk of intracranial hemorrhages. SCFN is generally reported in term or post-term infants in generally good health. Subcutaneous fat necrosis has been reported in term and post term infants requiring body cooling therapy following hypoxic ischemic encephalopathy. Most infants present with erythematous, violaceous plaques and nodules at one or more than one location. They are reported around shoulders, flanks, back and face. After the resolution of fat necrosis some infants continue to have cutaneous atrophy at the location of lesions. Subcutaneous fat necrosis has to be differentiated from conditions like chemical burns, neonatal sclerema, purpuric rashes secondary to sepsis or other etiologies for thrombocytopenia, cellulitis, scleredema, sclerema neonatorum and disseminated intravascular coagulopathy. Chemical burns can be seen secondary to application of chloraprep (chlorhexidine gluconate 2% and isopropyl alcohol 70%) or betadine prior to umbilical catheterization or procedures like lumbar puncture. Thrombocytopenia seen with congenital syphilis, systemic cytomegalovirus illness, toxoplasmosis, and congenital rubella can present with scattered petechiae and purpuric rashes. Most infants have associated intra-uterine growth delay and hepato-splenomegaly.10,11 Total IgM and torch titers are positive in infants with TORCH infections with severe thrombocytopenia. Neuro imaging may be required to rule out intracranial hemorrhages or calcification.
Scleredema has been reported in neonates, following exposure to the cold temperatures or those with severe diarrhea or infection during the first week of life. The involved skin is visibly indurated and waxy. The lower extremities are frequently involved but the skin is edematous and exhibits easy pitting.12 Majority of the infants have a mild illness and a full recovery.
Sclerema neonatorum is a diffuse induration of the skin in newborn infant.13,14 It manifests with firm tender skin lesions. The lesions are diffuse, sclerotic, usually distributed all over the body except palms, soles, genital and nipple area. It differs from SCFN, the lesions are rather diffused in presentation. Microscopically, lesions show intersecting fibrous bands with sparse lymphocytic infiltration. Histologically, there is no necrosis of fatty tissue, needle shaped crystals could be noted but there is absence of the infiltration of lymphocytes, histiocytes or multinucleated giant cells. The prognosis in sclerema neonatorum is much worse compared to SCFN.15 Sclerema is associated with significant morbidity and mortality. Intravenous immunoglobulin has been use to enhance humoral and cellular immunity and thus decrease the mortality associated with sclerema in a newborn.15 Subcutaneous fatty tissue in newborn infants have ample saturated fat, which tends to harden with hypothermia. Hypothermia with associated clinical shock may enhance subcutaneous hardening in sclerema neonatorum. The diagnosis is made clinically, based on diffuse skin hardening in a critically ill newborn. The involved skin is attached to the underlying tissue and cannot be folded, pinched, or pitted. Septic workup, complete blood counts, assessment of electrolyte balance and hydration status is of paramount importance. It is imperative to maintain normal skin temperature, avoid hypothermia, monitor urine output and serum electrolytes, and provide appropriate nutritional support. Routine antibiotics with a negative culture are not recommended. Use of intravenous immunoglobulin and systemic corticosteroids is debatable.
Purpura fulminans is a life-threatening serious disorder presenting with cutaneous hemorrhages and necrosis with associated disseminated intravascular coagulopathy. It has been reported with Staphylococcus, E coli, Enterobacter and other gram negative infections. It has been seen with deficiency of protein C and S. This disorder is associated with the lower concentration of fibrinogen, thrombocytopenia, prolonged prothrombin and partial thromboplastin times. Fever and leukocytosis could be present without presence of acute infection. Erythema is rapidly followed by irregular hemorrhagic necrotic lesions with surrounding erythema. Affected areas are tender and indurated.16,17
The exact etiology of SCFN is unknown. It could potentially be related to neonatal stress, hypoperfusion, hypoxemia, hypothermia, application of forceps or vacuum extraction, other instrumentation or aggressive resuscitation at delivery, meconium staining of amniotic fluid, maternal preeclampsia, maternal diabetes, maternal hypertension, and Rh incompatibility. Hypothermia or hypoxia may lead to subcutaneous fatty tissue to get crystallized with necrotic changes, accumulation of inflammatory infiltrates including macrophages and giant cells.18 The inflammatory cascade leads to release of 1,25 dihydroxy cholicalciferol resulting in excessive absorption of calcium from the intestines and excessive mobilization of calcium from bones with ensuing hypercalcemia and hypophosphatemia.19–21 Hypercalcemia has been reported in about 25-60% of cases of SCFN. Hypercalcemia can present prior to manifestation of cutaneous lesions or after the presentation of skin lesions. Hypercalcemia could be severe and may require withholding all supplementation of calcium with feedings or intravenous fluids. Biphosphantes may be recommended in stable term or post term infant with severe hypercalcemia.22 Persistence of hypercalcemia potentially could lead to nephrocalcinosis and acute renal injury. Intravenous hydration, use of furosemide and hydrocortisone are the common treatment options. We could not use IV furosemide in our infant because of associated hypernatremia and excessive insensible fluid losses. However, our patient was on hydrocortisone for hypotension. Prior to hydrocortisone therapy, the infant was on dopamine 5 microgram per kilogram per minute for less than twenty four hours. Relatively high normal serum calcium resolved in about seven days. No IV calcium was given during the period of relative hypercalcemia. Initially, the skin lesions presented with purplish discoloration of left flank with intact overlying skin. The skin had yellowish looking plaque by day four of life Figure 2 and the plaque gradually faded by week one of life. Mild elevation of temperature has been reported in some infants with fat necrosis. This may be related to elevation of prostaglandin E2 in some hypercalcemia patients. Elevation of serum interleukin 1 by fat necrosis has been reported.
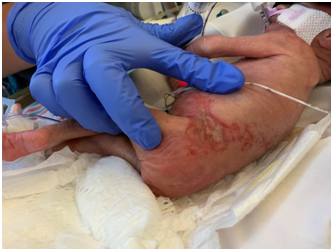
Figure 2 On Day four of life, there was partial resolution of the coloration of the plaque with yellowish deposits visible in the center.The overlying skin was intact.
Sepsis in a newborn infant can present with purpuric skin lesions. Broad-spectrum antibiotics may be recommended to rule out sepsis prior to culture report. The prognosis for subcutaneous fat necrosis in newborn infant is excellent. Majority of SCFN resolve spontaneously. Hypertriglyceridemia has been noted secondary to mobilization of fatty acid from the affected lipids tissue. There may be occasionally some atrophic scars on follow-up. Severe hypercalcemia potentially can be associated with failure to thrive, growth delay, emesis, seizures, shortening of QT interval, cardiac arrhythmias, renal failure, tissue calcification, hypertonia, and constipation.23 There have been some reported cases of hepatic and myocardial calcification.24 Anemia, hypoglycemia, hyperlipidemia and thrombocytopenia have been reported with SCFN. Our index patient had high normal serum calcium, hyperglycemia which was related to high glucose infusion rate associated with increased fluids to compensate for insensible fluid losses and hypernatremia. The infant was given few doses of IV insulin. Our infant required packed red blood cells, fresh frozen plasma and a platelet transfusion. Pamidronate has been used for subcutaneous fat necrosis with severe hypercalcemia.25 It is a bisphosphonate that alters bone formation and breakdown in the body. This can slow bone lysis and may help prevent bone fractures. Use of pamidronate for extremely preterm infants is not established. Hydrocortisone has been used for the management of hypercalcemia. Our index patient was on hydrocortisone for the management of hypotension. It may have possibly helped in early resolution of skin lesions and hypercalcemia.
Authors have no acknowledgments for this study.
The author declares that there are no conflicts of interest.
None.

©2019 Bharti, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 International Childhood Cancer Day is observed on 15 February 2026 to raise awareness about childhood cancers and to highlight the medical, developmental, and supportive care needs of affected children and their families. This day emphasizes the importance of early diagnosis, pediatric care, and continued research to improve survival and quality of life in children with cancer.
Researchers and healthcare professionals are encouraged to submit their original research articles, reviews, and clinical studies related to pediatric oncology, neonatal care, and child health. Manuscripts submitted on the occasion of International Childhood Cancer Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
.
International Childhood Cancer Day is observed on 15 February 2026 to raise awareness about childhood cancers and to highlight the medical, developmental, and supportive care needs of affected children and their families. This day emphasizes the importance of early diagnosis, pediatric care, and continued research to improve survival and quality of life in children with cancer.
Researchers and healthcare professionals are encouraged to submit their original research articles, reviews, and clinical studies related to pediatric oncology, neonatal care, and child health. Manuscripts submitted on the occasion of International Childhood Cancer Day will be eligible for a special publication discount of 30–40% in the Journal of Pediatrics & Neonatal Care (JPNC).
.