Journal of
eISSN: 2373-4426


Case Report Volume 4 Issue 6
1Hotel Dieu de France Hospital University Medical Center, Saint Joseph University, Lebanon
2Department of Pediatrics, University of Balamand, Lebanon
3Department of Pediatric Surgery, University of Balamand, Lebanon
4Department of Medicine, Saint Joseph University, Lebanon
Correspondence: Dany Al Hamod, Department of Pediatrics, Saint George University Hospital, University of Balamand, Beirut, Lebanon, Tel +961-368-3209
Received: May 20, 2016 | Published: June 29, 2016
Citation: Kamel R, Zeidan S, Aramouny E, Sabouneh M, Sacy R, Hamod DA (2016) Pyomyositis; A Diagnosis Not To Miss. J Pediatr Neonatal Care 4(6): 00163. DOI: 10.15406/jpnc.2016.04.00163
Pyomyositis is a primary bacterial infection of the skeletal muscles. It is known as tropical myositis as most cases have been reported in patients living in the tropics. However, the disease is increasingly being seen in non-tropical areas, especially in the United States, with little data reported in Lebanon. The disease has a predilection for large muscles especially the quadriceps and gluteal muscles. Pyomyositis is usually diagnosed late, leading to increased morbidity and mortality rates. Time of recognition of the disease directs the course of the treatment. Early diagnosis of pyomyositis, during the pre-suppurative phase, can be treated only with IV antibiotics without the need for surgical incision and drainage. We represent here the case of a 14 year-old male patient who presented with atypical symptoms of fever, dysuria, and abdomino-pelvic pain. Patient was early diagnosed with pomposities of the right obturator muscle with extension to form osteomyelitis of the symphysis pubis. Patient was solely treated for six weeks of Intravenous antibiotics (IV), with full recovery, without the need for surgical drainage.
IV, intravenous; WBC: white blood cell; CRP, C-reactive protein; MRI, magnetic resonance image; S. aureus: staphylococcus aureus
Pyomyositis is an uncommon and potentially life-threatening condition, characterized by a primary acute pyogenic infection of the skeletal muscles.1-3 It manifests as suppuration within skeletal muscles, forming mainly single or multiple abscesses. It was first reported by Scriba in 1885 as an endemic disease of the tropics,4 however, incidence of pyomyositis is increasing in non-tropical regions, especially in immune-compromised patients.5,6 Constituting approximately 35% of the reported pyomyositis cases, the pediatric population is an especially difficult subset of patients to diagnose.7,8 Diagnosis of pyomyositis in children is often delayed because of its vague presentation. A mean delay of 10 days from the onset of symptoms to the correct diagnosis has been reported in literature.8,9 Drainage of the muscle abscess, followed by administration of appropriate antibiotics, remains the mainstay of treatment, and usually leads to complete recovery.9,10
A 14 year-old male patient admitted to the hospital for fever and abdomino-pelvic pain. History goes back to 1 day prior to presentation when he started to present with acute onset sub-umbilical pain, aggravated by activity, and associated with high grade fever (39oC) and chills. Clinically, the patient was limping with restricted and painful movements of the hip. He reported pollakiuria and dysuria. No history of vomiting, diarrhea, or past medical history or any medication use. However, there is a history of trauma to his right big toe two weeks ago, with subsequent cellulitis. On physical examination, patient febrile 39°C, with blood pressure 120/70 mmHg. The patient was in moderate distress and appeared ill.
Cardiovascular exam revealed a regular tachycardia, no murmurs, rubs or gallops. His lungs were clear. The abdomen soft, with positive bowel sounds. Upon palpation, patient had right lower quadrant and supra-pubic tenderness, no rebound tenderness. There is a decreased range of motion in both hips with pain upon simple rotation of the hip. The skin exam did not reveal any local inflammation but there was a scar on the right big toe from a wound infection two weeks ago.
Laboratory blood tests showed a white blood cell (WBC) count of 12.2 x 103 cells/mm, neutrophil count of 74%, and lymphocyte count 9%, a C-reactive protein (CRP) of 17,6 mg/dl (normal <0.5 mg/dl). Urine analysis and abdominal ultrasound was normal. An urgent Abdominal and pelvic CT scan post oral contrast administration showed: increased density of the fat tissue just posterior to the anterior abdominal wall in the right inguinal area raising the possibility of torsion in the mesenteric fat tissue or appendicitis. However, no radiological signs of appendicitis were found (Figure 1).
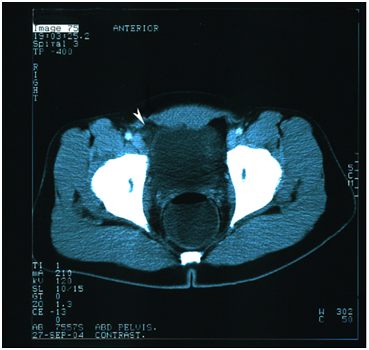
Figure 1 CT scan of the abdomen and pelvis with oral contrast showing increased density of the fat in the right inguinal region just posterior to the anterior abdominal wall.
Intravenous (IV) antibiotics were started (Ceftriaxone and Metronidazole) for coverage of Gram-negative and anaerobic bacteria, respectively. During the first 48 hours, the patient remained febrile, Teicoplanin added to cover Gram-positive bacteria and the patient started to show improvement with decrease in fever and pain. Laboratory studies also improved with WBC decreased to 4 x 103 cells/mm, CRP of 10 mg/dl, Erythrocyte sedimentation rate of 77 mm and Creatinine phosphokinase of 103 U/L (normal level between 24-190 U/L). Magnetic resonance image (MRI) of the pelvis revealed: Axial T1 W-FSPGR and coronal STIR sequence MRI a diffuse pelvic inflammation with extension into the inguinal canals mainly on the right compatible with pyomyositis (Figure 2). Blood culture grew Staphylococcus aureus sensitive to Cephalothin. So ceftriaxone and metronidazole were stopped and cephalothin was started. After five days of IV antibiotics, follow up blood tests showed WBC of 5.7 x 103 cells /mm, Neutrophils of 48% and Lymphocyte of 38%, CRP of 7mg/dl, and blood culture was negative as sterilization of blood stream has occurred. Repeated MRI of the pelvis on day 12 revealed two abscess cavities close to the right symphysis pubis, which shows high signal intensity on STIR sequence in favor of osteomyelitis (Figure 3).
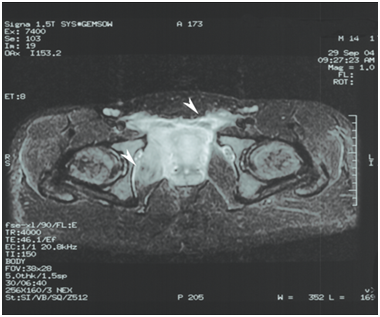
Figure 2 MRI of the pelvis, on the T1 sequence, showing abnormal high signal intensity on STIR sequence around the iliac and femoral vasculature sheath as well as in the right obturator interns muscle.
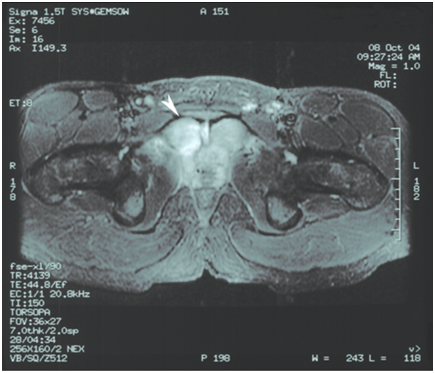
Figure 3 Pelvic MRI, T1 sequence, showing high signal intensity on STIR sequence involving the symphysis pubis compatible with osteomyelitis.
The patient received a total course of six weeks of IV antibiotics with marked improvement in his clinical and radiological signs. No surgical intervention or drainage required. Patient was discharged home on an additional two weeks of oral antibiotics, mainly fourth generation cephalosporin, without any clinical deterioration and favorable outcome.
Pyomyositis is an endemic disease in tropical regions; hence the name "tropical pyomyositis".4,10 Literature reports vary with respect to the most common site of muscle involvement in pyomyositis. Theodorou et al. documented that a solitary abscess in the quadriceps musculature is the most common pattern of the disease.11 According to Crum-Cianflone quadriceps and gluteus muscle groups are the most commonly affected sites.12 Bickels et al.13 noted that the quadriceps are the most usual place [26.3%] followed by pslliooas (14%).13 However, others have shown that the obturator internu is the most commonly affected (62% of patients) muscle group.14 Which is similar to our case? Patient was found to have two abscess cavities involving the obturator interns with extension to form osteomyelitis of thesymphysis pubis which explains the pollakuria and dysuria the patient had.
Pyomyositis is mainly caused by bacteraemia, as a result of a previous trauma or infection. Our patient indeed, reported a history of right big toe injury few weeks ago. Pyomyositis usually present, at the beginning, with limping and the inability to bear weight.15 The disease is classified into three stages.16 The first stage, or the invasive phase, occurs during the first two to three weeks after infection, with subacute presentation such as local swelling, mild pain, variable fever, and anorexia. During the second or suppurative phase, definitive abscess and pus are clearly evident. Findings at this stage could present as high fever, chills, local tenderness, and myalgia. The third and last stage of the disease is so severe which could even reach to toxic shock syndrome as reported by literature.17 In our case, the patient was in stage two of the disease where he presented with fever and severe hip pain with an evidence of abscess in the right obturator internus region. However, the symptoms of dysuria and pollakuria that the patient presented with were highly misleading. There are no reported cases of pyomyositis associated with similar symptoms. Urinary tract infection or appendicitis was highly suspected. Urine analysis was negative, thus a broader differential was then put including appendicitis, arthritis. Indeed, literature extensively reports that one of the main causes of delayed diagnosis, of pyomyositis, is that several differential diagnoses are possible, including tumors, osteomyelitis, muscle infarction, hematoma, muscle strain, thrombophlebitis, cellulites, septic arthritis, transient synovitis, sciatica, and epidural abscess.18-20 The only way to differentiate them is by imaging. In our case an urgent CT scan followed by MRI was ordered, which helped in the early diagnosis and management of the patient. The only explanation of the urinary symptoms, in our case, is irritation of the bladder by the inflammation due to proximity.
In pyomyositis usually laboratory data are not specific and could not lead to the diagnosis. Laboratory tests are generally nonspecific and of limited value. More specifically, white blood cell count is raised in 50% to 60% of cases and the erythrocyte sedimentation rate is also often elevated. The serum creatinine kinase levels are usually normal, as in our case.21 Eosinophilia is reported only in tropical cases and may be due to a parasitic cause of infection.20,21 Blood cultures are positive in only 5% to 30% of patients.22
Staphylococcus aureus (S. aureus) is the most common causative pathogen, affecting 90% of the patients in tropical areas and 60% to 70% of cases in temperate regions.23 Beta hemolytic group A Streptococcus is the next most common cause (Streptococcus pyogenes), followed by Escherichia coli.24 Methicillin-resistant S. aureus species have been increasing in frequency recently25 after its first detection in Singapore in 1996.25 For this reason, empirical antibiotic treatment was initiated (Ceftriaxone, Metronidazole and Teicoplanin), mainly for the coverage of gram negative, anaerobes and resistant S.aureus bacteria. However, when blood culture revealed S.aureus, sensitive to cephalothin, antibiotics were stopped and patient started on cephalothin, with marked clinical improvement after 48 hours of IV antibiotics.
Treatment of pyomyositis is usually in relation to the stage of the disease at the time of the diagnosis. In the early invasive stage, empirically administered antibiotics are considered the treatment of choice.26 In stages 2 and 3, percutaneous drainage or surgical incision and drainage of the pus formed, may be applied in combination with administration of 2 broad-spectrum antibiotics.27 When the pathogenic agent is isolated, antibiotic therapy should be adjusted with the results of culture and sensitivity tests. Optimal duration of treatment varies from 7 days of oral therapy to 6 weeks of intravenous antibiotic administration.28 In our case, although there was abscess formation in our patient, neither surgical nor percutaneous drainage was needed. Due to the risk of methicillin-resistant S. aureus in some countries, teicoplanin was prescribed as the first choice of antibiotic therapy in addition to a broad-spectrum coverage. Reports indicated that immunocompromised patients should be treated by broad-spectrum antibiotics, for targeting a wide-range of gram-negative and anaerobic pathogens29 However, Immune workup was not studied in our patient, due to the lack of evidence of any past medical history or recurrent infections that favors immunodeficiency. However, broad-spectrum antibiotic coverage was started until the pathogenic bacteria are isolated and patient was then switched to the specific antibiotic according to the sensitivity of the isolated bacteria. Patient improved within 48 hours of initiation of cephalothin, with a total of 6 weeks of IV-antibiotics, followed by another 2 weeks of oral antibiotics (fourth generation cephalosporin). Patient improved well with follow up MRI showing decrease in abscess size and, later, full recovery, with no need for any surgical drainage.
Pyomyositis is a rare, and yet, life-threatening infection worldwide, with increased incidence in temperate regions. No data yet is reported in Lebanon, with little recognition of the disease. Early recognition of pyomyositis, may change the coarse of the disease from life-threatening to complete recovery. Treatment is mainly antibiotic coverage, with or without drainage, of abscess when present. Physicians should start to be familiar with this disease in order to recognize and manage patients early so we can prevent major complications.
None.
None.

©2016 Kamel, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.