Journal of
eISSN: 2373-4426


Research Article Volume 10 Issue 5
1Unidad de Terapia Intensiva Pediátrica, Hospital Pediátrico de Sinaloa “Dr. Rigoberto Aguilar Pico”, México
2Centro de Investigación Aplicada a la Salud Pública, Facultad de Medicina, Universidad Autónoma de Sinaloa, México
3Hospital Civil de Culiacán, Centro de Investigación y Docencia en Ciencias de la Salud, Universidad Autónoma de Sinaloa, México
4Departamento de Investigación Hospital de la Mujer, Secretaría de Salud, México
5Departamento de Investigación, Hospital Pediátrico de Sinaloa “Dr. Rigoberto Aguilar Pico”, México
6Hospital Pediátrico de Sinaloa, Mexico
Correspondence: Jesús Javier Martínez García, Hospital Pediátrico de Sinaloa. Donato Guerra y Constitución S/N, Culiacán, Sinaloa México, CP 80200, Tel 6671 43 44 02
Received: October 23, 2020 | Published: October 27, 2020
Citation: Martínez-García JL, Gámez-Escarrega CJ, Martínez-Félix NS, et al. Plasma lactoferrin levels in newborn infants with early-onset neonatal sepsis. J Pediatr Neonatal Care. 2020;10(5):137-140. DOI: 10.15406/jpnc.2020.10.00426
Objective: To determinate if lactoferrin (LF) could be optimal biomarker; the LF levels in plasma were compared between neonates with or without early-onset neonatal sepsis (EOS).
Methods: From January to December of 2019 we conducted an analytic cross-sectional study, eighty-nine patients were collected: 34 newborns with EOS and 55 newborns without EOS from neonatology unit from a tertiary care hospital. The diagnosis was made with clinical parameters and sepsis biomarkers. Plasma concentrations of LF were measured by Enzyme-Linked Immunosorbent Assay (ELISA).
Results: The LF median was 3.7ng/ml to the newborn group with EOS while 44.8ng/ml was to newborn without EOS (p: 0.000). The best LF cut-off point in newborns with EOS was 20.55ng/ml, with sensitivity of 73.5%, specificity 92.7%, positive predictive value 86.2%, and negative predictive value 85%, positive likelihood ratio 10.11, and negative likelihood ratio 0.28. Conclusions: These findings indicate that levels of LF in plasma could be an effective and useful biomarker to diagnose of EOS.
Keywords: Lactoferrin; sepsis; newborn
Neonatal sepsis is a systemic infection caused by different microorganism (i.e. bacteria or fungi) occurring in infants at ≤ 28 days of life and is an important cause of morbidity and mortality of newborns.1 The incidence of this disease varies depending geographic regions, maternal and newborn risk factors, and prevention strategies.2,3 The reported incidence rate in the United States of America is one to four cases per 1,000 live births 4.5 while in Mexico the incidence rate can be highly variable, from 4 to 15.4 cases per 1,000 live births.4,5 On the other hand, is estimated that neonatal sepsis cause around 400,000 deaths per year worldwide.2,6 In fact, neonatal sepsis is the second leading cause of neonatal mortality, preceded only by complications related to preterm delivery.7
Neonatal sepsis has been classified as early onset or late onset, depending on the age of onset and the time of the sepsis episode.8 Early Onset Neonatal Sepsis (EOS) is defined as the appearance of signs and symptoms of sepsis within the first 72 hours and is acquired before or during delivery and generally represents a vertical transmission from mother to child, while late-onset infections (LOS) occur after delivery, or after 3 to 7 days of age, and are attributed to organisms acquired by interaction with the hospital or community environment.9 The mortality of EOS is higher than LOS; around the 35% of newly born die by EOS in comparison of LOS that 18% die.10 The correct diagnostic of sepsis is very important, for that it have been development different; complete white blood cell count with differential, a single blood culture, urine cultures, lumbar puncture for cell count and culture, C-reactive protein (CRP) and procalcitonin (PCT), of them blood culture being the gold standard.11-13 In case of EOS is not appropriate the use of blood culture due to the long incubation period to confirm the diagnosis, in addition, most blood cultures are negative in newborns with clinical signs of EOS.14 Aforementioned various markers have been studied for the diagnosis of EOS, such as C-reactive protein (CRP), procalcitonin (PCT), interleukin 6 (IL6), and more recent soluble differentiation group (sCD14) or presepsin.15,16 Despite the previously markers mentioned, the identification of more sensitive molecules that can diagnose early and timely EOS are necessary, such as immune-system proteins sensitive to infections, as is the case with lactoferrin.
Lactoferrin (LF) is an iron-binding protein that is produced and stored in specific granules of neutrophils and is secreted into tissues or blood in response to an inflammatory or infectious process.17 It has been observed that in children with septicemia with normal values of neutrophils, the plasmatic LF increase during the first day, whereas in septic children with neutropenia there is not increase of LF levels.18 In the newborn there is few studies and they show low plasma levels of LF in neonates with EOS with normal values of neutrophils, therefore, plasma levels of LF for some authors are considered as a highly sensitive and specific biomarker in newly born with suspected EOS.18-20 For the aforementioned the aim of this study was to measure the plasmatic levels of Lactoferrin among newly born with or without EOS and determinate if LF could be a biomarker to diagnose EOS.
A descriptive, cross-sectional, prospective and analytical study was carried out in the neonatology unit of the Hospital Civil de Culiacán Sinaloa, México. Center for Research and Teaching in Health Sciences, and Center for Research Applied to Public Health of the Autonomous University of Sinaloa. The study was approved by the research ethics committee with registration number 023 and informed consent by parents. All newborns who were hospitalized from January to December 2019 in the Neonatal Intensive Care Unit (NICU) of the Civil Hospital of Culiacán Sinaloa were included in the study. Neonates with suspected neonatal sepsis had at least one of the following signs and symptoms: fever or hypothermia, respiratory distress, cyanosis or apnea, poor tissue perfusion, lethargy or irritability, refusal to feed, hypotonia, seizures, bulging fontanelle, signs of bleeding, bloating, hepatomegaly, diarrhea, bloody stools and unexplained jaundice.
Blood samples were taken during the first 10 hours of life, for: leukocytes, neutrophils, platelets, C-reactive protein, procalcitonin levels, and blood culture. Newborns studied had to be fasting at the time of taking the blood sample. Newborn with EOS was considered to be one with the described signs and symptoms, a positive blood culture, or in the case of a negative blood culture having serum procalcitonin levels ≥8.5ng/ml. The control group was newborns with non-infectious pathology, negative blood culture, or serum procalcitonin levels ≤ 8.5ng/ml. Plasmatic LF levels were measured by IgG class autoantibodies against lactoferrin by ELISA (Bovine Lactoferrin, ELISA Quantitation Set, Bethyl Laboratories. Montgomery, TX. USA).
The normality of the data was performed by the Komogorov Smirnov test. Continuous variables with normal distribution were presented by means and standard deviation (SD), and comparisons were made with student's t for independent samples. Continuous variables with non-normal distribution were presented by medians and interquartile range (IQR), and comparisons of two groups with non-normal distribution with the Mann Whitney test. Categorical variables were expressed as numbers and percentages. Categorical data were compared using the X2 test or Fisher's exact test. The correlation between the numerical variables was performed with the Spearman's Rho correlation coefficient. The operative characteristics of the receptor (OCR) and the area under the curve (AUC) were carried out to evaluate the diagnostic capacity of neonatal sepsis by LF. Sensitivity, specificity, prediction values, likelihood ratios and 95% confidence intervals (95% CI) were calculated. Statistical analysis was performed with SPSS version 22 software. p ≤0.05 value was considered significant.
Of total 89 newly born that were hospitalized in NICU, 38.2% (34/89) were diagnosed with EONS, of then the 8.8% (3/34) had positive blood cultures for staphylococcus aureus while 61.8% (55/89) were newly born without EOS. The medina of plasmatic LF with EOS was 3.76 ng/mL (IQR: 0.15-24.10 ng/mL) lower that newly born without EONS with 44.76 ng/mL (IQR 15.15-82.12 ng/mL, p: 0.000) as shown Figure 1. On the other hand, the best cut-off point for plasmatic LF in patients with EOS was 20.55 ng/mL, the area under the curve was 77.8% (95% CI: 67.9-87.6%, p: 0.000), Figure 2. The observed sensitivity was 73.5%, (95% CI: 58.7-88.35%); specificity 92.72%, (95% CI: 85.86-99.59%). The positive predictive value was 86.20%, (95% CI: 73.65-98.75%) and negative predictive value was 85.0%, (95% CI: 75.96-94.0%) The positive Likelihood ratio was 10.11, (95% CI: 3.8-26.53) and negative Likelihood ratio was 0.28, (95% CI: 0.161-0.504).
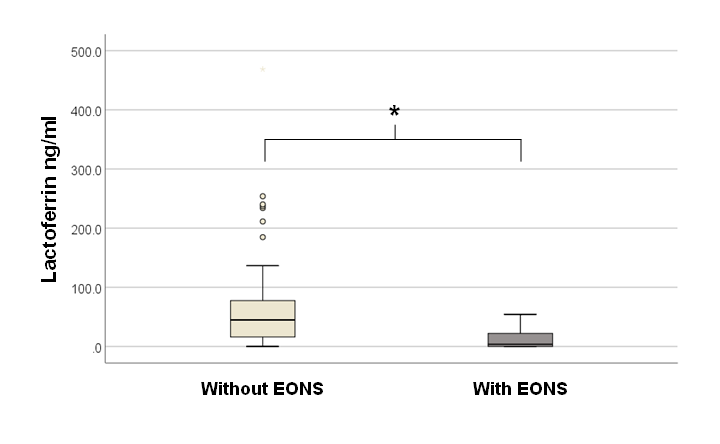
Figure 1 Tukey's graph, decreased plasma LF levels in neonates with early neonatal sepsis (EONS). *: p 0.000.

Figure 2 Receiver operating characteristics (ROC) curve to determine the diagnostic capacity of plasma LF levels in detecting early neonatal sepsis.
Other characteristic parameters of sepsis were measured in both groups, such as platelets, the median to patients with EOS was 88500 mm3 (IQR: 158750-279750 mm3) these levels were lower in comparison with patients without EOS with 235000 mm3, (IQR: 201500-283500 mm3, p: 0.017). Procalcitonin also was quantified, the median to group with EOS was 14.11 ng/mL (IQR: 10.9-27.1 ng/mL) while the median to group without EOS was lower 1.27 ng/mL (IQR: 0.45-3.56 ng/mL, p: 0.000). Other parameter compared between both groups was the absolute values of neutrophils in which there was not statistical difference among the study groups.
In addition, it was searched a correlation between plasmatic levels of LF and procalcitonin, due to this protein is an important indicator of sepsis; the correlation was moderate, around 46% (p: 0.000) as shown in Figure 3. On the other hand, no correlation was observed between no correlation was observed between absolute values of neutrophils of both groups and plasmatic levels of LF (p: 0.339) as shown the Figure 4.
Early-onset sepsis remains a common and serious problem for neonates, especially preterm infants. Group B streptococcus (GBS) is the most common etiologic agent, while Escherichia coli is the most common cause of mortality.21 Unfortunately, in this case of disease is not convenient the use of blood culture to detect these pathogens for test time and is necessary a higher concentration of bacteria.22 It have been identified of diverse biomarker to give a correct treatment combat EOS and avoid than increase the mortality rate in neonates such as white blood cell counts, CRP, PCT, interleukin 6 (IL-6), interleukin 8 (IL-8), gamma interferon (IFN-), and tumor necrosis factor alpha (TNF-), and cell surface antigens, including soluble intercellular adhesion molecule (sICAM) and CD64, among others.23-25 Research to find an optimal biomarker that, together with clinical signs, can make easier and faster the EONS diagnosis. In this study we propose the plasmatic levels of lactoferrin an option such as efficient biomarker.
Plasma concentrations of LF in the newborn are related to gestational age, lower in preterm infants compared to term infants. Scott1 et al., (1989) carried out a prospective study in 23 preterm infants between 24 and 36 weeks of gestational age with the objective of evaluating the plasma levels of LF in infection. Plasma LF levels were lower at lower gestational age, and an increase in LF levels was observed in infected patients. The LF range in this study was 50 to 900 μg/L (50-900ng/mL).20 In our research we did not observe a difference in plasma LF levels at different gestational ages, and in the group of newborns with EOS the levels were lower than in the group of newborns without EOS.
Decembrino et al., (2017) evaluated plasma LF levels in a very small sample of 15 newborns older than 72 hours of life with suspected infection. The serum LF concentration was significantly lower in septic infants. The area under the curve was 90% with 95% CI: 63 to 99%. The best cut-off point for LF was <1.2μg / mL with a sensitivity of 100% and a specificity of 81.8%. They observed a positive correlation between serum LF values with total leukocytes and neutrophil count. Our results observed similarities with Decembrino et al. despite we obtained in a larger sample (Figure 1 and 2).
The plasma levels of LF in the premature newborn not only depend on the number of neutrophils, it also depends on the LF content in the neutrophils, the degranulation characteristics of the neutrophil, and the life of the LF in the plasma.26 The degranulation capacity is similar between the neutrophils of the term and adult neonates, whereas in preterm infants, there is deterioration in the release of bactericidal/permeability-increasing protein, elastase and lactoferrin compared to neonatal cells of term or adults.27 LF levels in neutrophils of term neonates were half the concentrations of adults, while in neutrophils of preterm infants they had lower LF concentrations.20,27,28 In our study, we did not observe a direct correlation between the absolute number of neutrophils and plasma lactoferrin levels (Figure 4).
On the efficiency of plasma LF levels in newborns with EOS, but we did not observe a correlation between plasma LF levels and total count leukocytes and total neutrophils. Ahmed et al., (2019) carried out a prospective and comparative study in the NICU of a University Hospital in Cairo, Egypt, comparing the diagnostic value of presepsin, procalcitonin, c-reactive protein, lactoferrin, interleukin 6 (IL-6), interleukin 8 (IL- 8). Presepsin was the most efficient biomarker with a sensitivity of 88.9%, specificity of 85%. LF was the biomarker that showed the lowest diagnostic capacity of EONS with sensitivity 55.6% and specificity 64.3%.15 In our research, unlike the previous study, we observed that plasma LF levels have an acceptable efficacy and utility for the diagnosis of EOS.
Our study has some limitations such as; the sample size is limited in patients with EOS, although the purpose of increasing the number of patients without EOS was to improve statistical efficiency. The gold standard for EOS was not the blood culture as in other studies, only three blood cultures were positive, therefore, the gold standard that we used was limited to clinical parameters and to another sepsis biomarker with acceptable sensitivity and specificity and finally, a single plasma LF measurement limits the correct interpretation of the results obtained, therefore, longitudinal cohort studies with larger sample sizes are necessary.
In conclusions the group of newborns with EONS, they had a lower plasma concentration of LF compared to newborns without EOS, therefore, plasma LF levels in our study were an effective and useful marker for the diagnosis of EOS.
The specific contribution of each author to the study
Jesús J Martínez-García: Methodological and statistical analysis
Claudia J Gámez-Escarrega: Information analysis and laboratory sampling
Nora S Martínez-Félix: Information analysis and laboratory sampling
Adrián Canizalez-Román: ELISA test processing and Methodological analysis
Uriel A Angulo-Zamudio: ELISA test processing and translation of the article into English
Nidia M León-Sicairos: ELISA test processing and Methodological analysis
Centro de Investigación Aplicada a la Salud Pública, Facultad de Medicina. Universidad Autónoma de Sinaloa. Hospital Civil de Culiacán. Centro de Investigación y Docencia en Ciencias de la Salud.
None.
None.

©2020 Martínez-García, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.