Journal of
eISSN: 2373-4426


Research Article Volume 11 Issue 1
Mother and Child Care Research Institute, Russia
Correspondence:
Received: February 16, 2021 | Published: February 22, 2021
Citation: Kosovtsova N, Pavlichenko M, Bashmakova N. Outcomes of intrauterine interventions for the treatment in fetal urinary tract obstructions. J Pediatr Neonatal Care. 2021;11(1):7-13. DOI: 10.15406/jpnc.2021.11.00434
Introduction: The leading cause of chronic renal failure in newborns, which occurs soon after birth is obstructive uropathies.
Objectives: To prove the effectiveness of nephroamniotic shunting based on the evaluation of perinatal outcomes of this procedure, as well as a comparative analysis of the use of the stent manufactured by "Cook" (Ireland), 3.0 Fr/100 mm and the stent “SDE–MED”, 3.0 Fr/50 mm with the original shape of pigtails.
Methods: After checking the safety of the stent “SDE-MED”, 3.0 Fr/50 mm developed by FGBU Mother and Child Care Research Institute with the original shape of pigtails in an animal experiment, the stent was used in clinical practice for intrauterine shunting in cases of unilateral or bilateral hydronephrosis of grade III to IV and posterior urethral valve disorder. In the final part of the study, a comparison of the outcomes of nephroamniotic shunting using two different stents was made.
Results: The “SDE–MED” 3.0 Fr/50 mm stent provided more effective fixation in the fetal kidney cavity system in comparison to the stent manufactured by “Cook” (Ireland) 3.0 Fr/100 mm.
Conclusion: Intrauterine shunting surgery of the upper urinary tract is a pathogenetically justified method of treating urinary tract obstructions and can be used to correct all types of fetal renal obstructions.
Thanks to prenatal ultrasound, the number of urinary tract obstructions diagnosed before birth has been increasing in the last decade.1 The share of congenital defects of the urinary system, including urinary tract obstruction, reaches up to 65% in chronic kidney failure in children.2 The variability and high prevalence of fetal urinary tract defects, cause to consider the problem of the prevention of their complications. Experimental models of ureteral or urethral obstruction in fetal animals resulted in renal dysplasia, and intrauterine decompression of the upper or lower urinary tract prevented abnormal renal differentiation.3 Experimentally, it was demonstrated that the kidney function of newborn lambs was directly proportional to the duration of intrauterine decompression and inversely proportional to the duration of obstruction. Changes in the structure of the renal parenchyma and the kidney function of experimental animals persisted after birth and directly depended on the duration of urinary tract obstruction.4 Interstitial fibrosis of the renal parenchyma and renal atrophy are the final stages of long-term ureteral obstruction.5
The presence of fetal infravesical obstruction or bilateral hydronephrosis of grade III to IV in combination with oligohydramnios is a prognostically unfavourable sign for the life and health of newborns, accompanied by the formation of lung hypoplasia, cystic kidney dysplasia, and urinoma. These complications of obstructive uropathy can be prevented by intrauterine correction. Data from the study of functional parameters of kidneys based on pre and postnatal observation of 452 patients who had congenital malformations of the urinary system by a large perinatal centre of the Ural Federal District, Russia from 2014 to 2019 showed that 36 patients (8%) with obstructive kidney damage in fetuses had a bilateral nature and were accompanied by oligohydramnios, which indicated a decrease in kidney function at the intrauterine stage of the child’s development. Data from other studies confirm that newborns who were diagnosed in utero with a posterior urethral valve disorder (PUV) were at high risk (32-50%) of antenatal fetal death due to lack of kidney function,6 and in infants who were found to have hydronephrosis of grade III to IV, there is a threat of urinoma formation and loss of organ function.7
Vesicoamniotic shunting for infravesical obstruction is the most frequently used method of intrauterine correction. The literature contains rare references to intrauterine shunting or drainage of urinoma.7 The most common indications for intrauterine vesicoamniotic shunting are PUV disorder and urethral atresia. In 2007, a multicentre randomised controlled trial of lower urinary tract obstructive lesions (Percutaneous shunting for Lower Urinary Tract Obstruction [PLUTO]) had been attempted in Europe. The scientific trial was discontinued, but the preliminary results showed that prenatal vesicoamniotic shunting improves perinatal outcomes in infravesical obstruction by reducing renal parenchyma compression and preventing lung hypoplasia.8,9 Other reports of positive results of intrauterine use of vesicoamniotic shunting for infravesical obstruction in fetuses are rare and do not provide convincing evidence of the benefits of this method.8,9 Nephroamniotic shunting is an even more rare fetal procedure designed to decompress the renal pelvis. There is extremely few information on the use of nephroamniotic shunting in the existing scientific literature.7
Objectives of the study
To prove the effectiveness of nephroamniotic shunting by evaluating perinatal outcomes after this procedure and to conduct a comparative analysis of the use two stents which are intended for shunting fetal kidneys for the purpose of surgical correction of obstructive lesions of the urinary tract. The stents, "Cook" 3.0 Fr / 100 mm stent and the “SDE–MED” 3.0 Fr/50 mm stent developed by FGBU Mother and Child Care Research Institute, differ in design of the end fixing elements - pigtails.
The procedure of nephroamniotic shunting under ultrasound control was initially performed on laboratory animals (lambs): 3 animals were installed with neonatal stents manufactured by the company "Cook" 3.0 Fr, 10 mm and the other 3 animals were installed with stents developed by FGBU Mother and Child Care Research Institute "SDE-MED" 3.0 Fr/50mm with the original pigtail shape (Patent no.) (Figure 1&2). This experiment was approved by the Ethics Committee No. 5 of FGBU Mother and Child Care Research Institute of the Ministry of Health of the Russian Federation on 10.10.2012.

Figure 1 Curl of the stent 3.0 Fr / 10 cm, by manufacturer "Cook", Ireland. The catalogue of the company "Cook".

Figure 2 General view of the stent "SDE-MED", developed by FGBU Mother and Child Care Research Institute of the Ministry of Health of the Russian Federation.
Method of nephroamniotic shunting
A G16 needle was used as a guide for inserting the stent into the kidney. The puncture needle was placed in the fetal renal pelvis with ultrasound navigation. A stent attached on a conductor was passed through the needle into the renal pelvis. The stent was installed by an external pusher in the collecting system of the kidney until the proximal pigtail was twisted. Then the needle was withdrawn into the amniotic fluid, and the remaining part of the stent was pushed through. The second end of the stent should be visualised in the amniotic cavity during ultrasound control after the stent was installed. In case of anhydramnios or oligohydramnios in the fetus, amnioinfusion of saline solution was performed before nephroamniotic shunting in order to improve visualisation and create a sufficient vertical pocket of amniotic fluid for nephroamniotic shunting.
The total number of installed stents was 6 (1 device for each animal). The duration of intrauterine life varied from 75 to 98 days. Another similar experiment was carried out in animals that had 110 to 120 days of intrauterine life. 21 days after the shunting procedure, the animals were removed from the experiment, and their kidneys were studied by organometry, overview microscopy of the slide mounts, and morphometry. Intact contralateral kidneys were used for comparison as a variant of the physiological norm.
Later, nephroamniotic shunting was introduced into the clinical practice of the FGBU Mother and Child Care Research Institute, Yekaterinburg, Russia. 40 (n=40) pregnant women with obstructive uropathies present in their fetuses were monitored. The women were divided into two groups: the main group and the control group. The main group included 26 pregnant women (n=26) with obstructive malformations of the fetal urinary system. For the purpose of intrauterine correction of the malformations, they underwent nephroamniotic shunting. The control group (n=14) included pregnant women whose fetuses also had obstructive kidney diseases, but they were not subjected to fetal renal shunting. The main group were also divided into two clinical subgroups, depending on which stent device was used for correction: in 14 pregnant women (n=14) with obstructive uropathies present in fetuses, the stents 3.0 Fr/100 mm, manufactured by "Cook" were installed (from 2012 to 2015) and in 12 pregnant women (n=12), the stents "SDE – MED" 3.0 Fr/50mm were installed (from 2016 to 2018). The material selected for manufacturing the “SDE-MED” stents was silicone rubber compound MC series and MS-R-STP MS No. 1-93. The silicone tube had an outer diameter of 1.05±0.03 mm and an inner diameter of 0.55±0.03 mm. (Patent No. 2459583 of 2011), developed by the FGBU Mother and Child Care Research Institute of the Ministry of Health of the Russian Federation. (This study was approved by the Ethics Committee No. 6 of the FGBU Mother and Child Care Research Institute of the Ministry of Health of the Russian Federation on 17.10.2012).
Ultrasound Indications for prenatal nephroamniotic shunting:
Indications for surgery:
The main purpose of intrauterine surgical correction of obstructive uropathy in fetuses is the preservation of organ function and prevention of the occurrence of secondary renal failure and lung hypoplasia.
Contraindications to intrauterine surgery:
Anaesthesia and fetal immobilisation
In order to immobilise the fetus, pipecuronium bromide was introduced into the umbilical vein (arduan, Reg. No.: П N011430/01 dated 24.03.09) at a dose of 0.1 ml per 1 kg of fetal weight.
In order to anesthetise the fetus, fentanyl citrate was injected into the umbilical cord vein at a dose of 10 mg/kg of fetal weight. (Reg. No.: PN000266/01 dated 10.10.11). During the procedure of nephroamniotic shunting on a pregnant woman, prophylactic antibiotics were administered. Parenteral administration of the antibiotic was performed 30 minutes before the operation. The control group consisted of 14 pregnant women with similar obstructive malformations of the urinary system in fetuses who did not undergo intrauterine interventions (n=14).
Patients were consulted on all treatment options, including wait-and-see tactics, and possible repeated renal pelvis shunting. All the patients issued written informed consent to a medical procedure for shunting of the fetal kidneys.
For indicators of qualitative traits, the absolute value and relative value was indicated in per cent, the critical level of significance of differences (p) at which the null hypothesis of no differences was rejected and the alternative was accepted, was set to 0.05. All statistical analyses were performed using SPSS 22.
Results of an experimental study
In order to identify the possible damaging effects of the shunting procedure on the kidney tissue of experimental animals (n=6), additional research methods were carried out. In the kidneys shunted using “Cook” stents and “SDE-MED” stents, the fibrous wall of the wound canal in the parenchyma occupied 0.95±0.09% of the kidney area. The leukocytic infiltration in the wall of the wound canal was only 0.18±0.07% of the volume of the kidney parenchyma. The proportion of the lumen of the wound canal was 2.58±0.02%.10,11 In the first 3 fetuses, “Cook” neonatal stents were found in the renal pelvis in 2 cases, and in 1 case, intrauterine stent expulsion had occurred. In the other 3 fetuses installed with the stent "SDE–MED", no expulsions were observed (Figure 3).
According to the results of the experiment, it was concluded that the overall condition of the shunted kidney parenchyma did not differ from the kidney tissue in the control group. Morphometry of fetal kidneys of the experimental animals after intrauterine shunting proved the safety of using nephroamniotic shunting and the use of the developed nephroamniotic stent "SDE-MED" 3.0 Fr/50mm.
Results of clinical application
After adapting the method of fetal kidney shunting on experimental animals, the method was introduced into clinical practice. The total number of pregnant women with obstructive fetal uropathies was 40 pregnant women whose fetuses had obstructive uropathies (Figure 4).
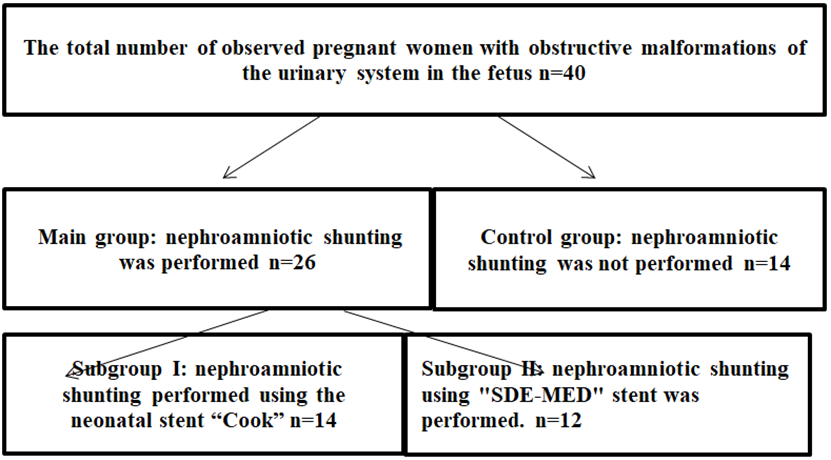
Figure 4 Distribution of pregnant women with obstructive urinary tract malformations in fetuses by groups.
The main group (n=26) was represented by patients with the following types of urinary outflow obstruction: infravesical obstruction in 7 cases (26.9%); unilateral hydronephrosis in 10 cases (38.5%); bilateral hydronephrosis in 9 cases (34.6%). The control group was represented by fetal patients with infravesical obstruction in 4 cases (28.6%); unilateral hydronephrosis in 8 cases (57.1%); bilateral hydronephrosis in 2 cases (14.3%) (Figure 5). 62% of pregnant women in the main group, showed oligohydramnios ranging from anhydramnios (PUV disorder - 26.9%) to moderate oligohydramnios in bilateral hydronephrosis. In case of unilateral hydronephrosis, the normal amount of amniotic fluid was determined. In the control group (n=14) anhydramnios was observed in 4 cases, with infravesical obstruction (28.6%). Oligohydramnios was observed in 6 cases of bilateral hydronephrosis (42.8%).
The groups were comparable in terms of somatic anamnesis, obstetric anamnesis and the spectrum of obstructive fetal malformations (Table 1).
Groups |
Bilateral hydronephrosis, n, % |
Unilateral hydronephrosis, n, % |
Infravesical obstruction, n, % |
Total, n |
р |
Main Group |
9 (34,6) |
10 (38,5) |
7 (26,9) |
26 |
р>0,05 |
Control Group |
2 (14,3) |
8 (57,1) |
4 (28,6) |
14 |
Table 1 Characteristics of obstructive lesions in fetuses of the main group and the control group
In order to exclude chromosomal pathology, fetal karyotyping was performed in all cases, and a normal karyotype was registered.
The consistency of the renal parenchyma before shunting was determined by following methods:
With normal parameters of fetal urine analysis, blood flow in the renal parenchyma was mostly preserved. The examination was performed using power Doppler mode with maximum magnification of the fetal kidney (the kidney should occupy ½ of the screen, PRF 0.6 kHz). In our study, the assessment of blood flow in the renal parenchyma before shunting surgery had a sensitivity of 87% and specificity of 92%.The gestational age of children born in the main group with nephroamniotic shunting was 38.9±1.1 weeks, and in the control group, the gestational age was 36.6± 4.7 weeks [p >0.05]. Natural birth was observed in the main group in 84.6% of the cases, and in the control group in 71.4% of the cases [p >0.05]. In 11.5% of the cases, postnatal surgical correction was not required for newborns. In 1 case (3.8%), a newborn from the main group died after 7 hours of life from respiratory failure caused by primary lung hypoplasia, which was confirmed by a pathological and anatomical examination. Mortality in the comparison group was 21.4 % of patients due to prenatal mortality [p<0.05]. Thus, the efficacy of intrauterine treatment was 96.2% (Table 2).
Characteristics |
Main Group |
Control Group |
р |
Perinatal Outcomes |
|||
Gestational period of the first |
24,8±2,7 |
- |
|
Gestational age of children born (weeks) |
38,9±1,1 |
36,6±4,7 |
р >0,05 |
Birth though natural ways (%) |
84,6 |
71,4 |
р >0,05 |
Postnatal Outcomes |
|||
Mortality (%) |
3,8 |
21,4 |
р <0,05 |
No indication for postnatal correction |
11,5 |
0 |
р <0,05 |
Lung hypoplasia (%) |
3,8 |
7,1 |
р <0,05 |
Chronic kidney disease (%) |
7,6 |
21,4 |
р <0,05 |
Table 2 Data on the results of nephroamniotic shunting
Children who received intrauterine interventions for various types of obstructive uropathies were monitored by a paediatrician, nephrologist, and paediatric surgeon during the first year of life. In our clinical practice, the use of prenatal nephroamniotic shunting surgery significantly reduced postnatal complications from 49.9% to 19% due to reduced mortality, the frequency of lung hypoplasia and chronic kidney disease. [p<0.05]. Two (7.6%) cases of the formation of chronic kidney disease of the first stage (Classification of chronic kidney disease, National Kidney Foundation/Kidney Disease - Outcomes Quality Initiative, 2002) were noted in the main group of patients during the first year of life, which was characterised by the signs of kidney damage without a reduction in the glomerular filtration rate. In the control group, the number of cases was higher and amounted to 21.4% [p<0.05], the severity of the disease was at stages I to III. There were no indications for peritoneal dialysis or kidney transplantation in all groups of patients.
The next stage of this multi-level cohort study was a comparative analysis of the use of stents of two different designs: 3.0 Fr / 100 mm, manufactured by “Cook", Ireland and the stent "SDE-MED" 3.0 Fr/50mm with the original form of pigtails developed by FGBU Mother and Child Care Research Institute. For this purpose, 2 subgroups were identified in the main group of 26 pregnant women. Subgroup I included 14 pregnant women with obstructive congenital malformations of the fetus, who underwent intrauterine shunt operations with a neonatal stent 3.0 Fr/100 mm, manufactured by "Cook", Ireland (n=14). Indications for intrauterine intervention in these patients in 6 cases (42.8%) was bilateral hydronephrosis of grade III to IV, in 5 (35.7%) cases there was unilateral hydronephrosis of the grade III to IV. In 3 (21.4%) cases, renal shunting surgery was performed for PUV disorder.
The remaining 12 pregnant women with obstructive congenital malformations of the fetus formed Subgroup II, in which fetuses underwent intrauterine shunting surgery using the stent "SDE-MED" 3.0 Fr/50mm (n=12): infravesical obstruction in 4 cases (33.3%); unilateral hydronephrosis in 8 cases (41.7%); bilateral hydronephrosis in 2 cases (25%).
The average gestational period of fetuses at the time of nephroamniotic shunting using the “Cook” stent was 24.8 ± 2.7 weeks whereas in fetuses using the “SDE-MED” stent it was 23.2± 1.4 weeks [p>0.05]. In 85.8% of patients with "Cook" stents installed, shunting surgery was performed repeatedly, as stent expulsions into the amniotic cavity were observed. In patients where the SDE-MED stent was used, repeated stent placement operations were performed in 13.3% of cases and were caused by stent dislocation in the amniotic cavity [p<0.05]. When the stent was expulsed, the shunting procedure was repeated until 34 weeks of pregnancy. The minimum stay of the “Cook” stent was 7 days, and the maximum was 48 days (M±m = 15.4±10.1). The minimum stay of the “SDE-MED” stent in the renal pelvis was 22 days, and the maximum was 122 days (M±m = 64.7±, 15, 2) [p <0.05] (Table 3).
Group Number |
Subgroup I |
Subgroup II |
р |
Prenatal outcomes |
|||
Gestational age at first shunting (weeks) |
24,8±2,7 |
23,2±1,4 |
р >0,05 |
Stent expulsions (%) |
85,8 |
13,3 |
р <0,05 |
Minimal stay of stent in the kidney (days) |
7 |
22 |
р <0,05 |
Maximum stay of stent in the kidney (days) |
48 |
122 |
р <0,05 |
Gestational age at birth (weeks) |
38,8±1,1 |
39,2±1,0 |
р >0,05 |
Natural childbirth (%) |
84,6 |
86,1 |
р >0,05 |
Postnatal Outcomes |
|||
Mortality (%) |
7,1 |
0 |
р <0,05 |
Postnatal surgical correction not performed (%) |
0 |
25 |
р <0,05 |
Lung Hypoplasia (%) |
7,1 |
0 |
р <0,05 |
Chronic kidney disease (%) |
7,1 |
8,3 |
р >0,05 |
Table 3 Data on the results of nephroamniotic shunting surgery using the “Cook” neonatal stent and the “SDE-MED” stent
Thus, it was found that the only complication of intrauterine fetal kidney shunting was stent expulsions. The expulsion of the “Cook” stents was explained by the imperfection of the mechanism for fixing these devices in the fetal body cavities against the background of extremely mobile fetal body, while the “SDE-MED” stents were reliably fixed in the fetal urinary tract and were less susceptible to dislocation. Fetal kidney shunting using the "SDE-MED" 3.0 Fr / 50mm stent allowed to achieve a positive effect associated with the restoration of urodynamics in the antenatal period, which did not require postnatal surgical correction of obstructive uropathy in 25% of newborns (in 3 patients with unilateral and bilateral hydronephrosis). Hypoplasia of the lungs and mortality in patients with "Cook" stents was explained by premature dislocation of the stent and its shorter stay in the renal cavity system.
Figure 5,6 shows a case of bilateral nephroamniotic shunting surgery with the "SDE-MED" stent in case of PUV. Stent expulsions were not observed. Shunting surgery of the left fetal kidney was performed at 22 weeks of pregnancy, after 500 ml of amnioinfusion, and the shunting surgery of the right kidney was performed at 24 weeks of pregnancy. During the first procedure of intrauterine shunting, there was pronounced oligohydramnios, and the amniotic fluid index was equal to 4 cm, which required amnioinfusion in the volume of 500 ml. Indications for shunting surgery of the right kidney were grade III hydronephrosis, which did not stop after shunting surgery on the left kidney. Postnatally, the function of both kidneys was preserved. After surgical correction of the PUV disorder, the patient currently has pyelectasia in the left kidney.
Figure 7 shows a case of nephroamniotic shunting for unilateral hydronephrosis of grade III to IV in a fetus at 22 weeks of gestation. The "SDE-MED" stent remained in the left kidney of the fetus for 17 weeks, and 6 days, delivery was performed through the natural birth canal, the stent was removed after the birth of the child in the maternity ward, and postnatal correction of the urinary system defect was not required.
This study demonstrates the introduction of a minimally invasive method of fetal kidney shunting into clinical practice using a new stent with the original form of pigtails which provides a more reliable fixation in the renal cavity system. The frequency of stent displacement decreased from 85% to 13.3% [p<0.05]. It was found that intrauterine shunting of the renal cavity system is a pathogenetically justified method of treatment and is applicable for all types of obstructive pathology of the urinary system in the fetus. According to the results of scientific work, the use of intrauterine nephroamniotic shunting surgery in clinical practice significantly reduced postnatal complications from 49.9% to 19% [p<0.05]. The occurrence of chronic kidney disease in the postnatal period decreased from 21.4 to 7.6%, and the mortality rate also decreased by 17.6 %.
Shunting of fetal kidneys using the stent "SDE-MED" 3.0 Fr /50mm allowed to achieve a positive effect associated with antenatal restoration of urodynamics. Consequently, the need for postnatal surgical correction of obstructive uropathy was not required in 25% of cases (in 3 patients with unilateral and bilateral hydronephrosis). Thus, it can be assumed that the outcome in patients without the use of nephroamniotic shunting would have been unfavourable since hydronephrosis of grade III to IV was registered in the period of 19-20 weeks, renal parenchyma was 2 mm, blood flow in the parenchyma was sharply reduced. The data from this multi-level cohort study are consistent with the results of other rare studies of prenatal treatment of obstructive uropathies in fetuses. In 2007-2012, Morris R. K., Ruano R., Kilby M. D. attempted a multicentre, randomised, controlled trial (Percutaneous shunting for Lower Urinal Tract Obstruction randomised controlled trial [PLUTO]).12-14 The aim of the study was to determine the effectiveness and acceptability of vesicoamniotic shunting surgery in fetuses with infravesical obstructions (LUTO). The study involved pregnant women with a male fetus with LUTO. However, this study was discontinued due to difficulties in randomisation. Based on the analysis of 31 cases of obstructive urinary tract malformations in fetuses, survival up to 28 days after the use of vesicoamniotic shunting was higher (50%), than with conservative management (27%). Twelve-month survival was 44% with vesicoamniotic shunting and 20% with conservative management. Based on the results obtained, it was concluded that after intrauterine correction by vesicoamniotic shunting, the survival rate of children within 28 days and 12 months is higher than with conservative management, but it is impossible to clearly prove the benefit of these operations unambiguously. Unfortunately, the prognosis for live birth and recovery of kidney function was unfavourable.12
The results of our study demonstrated a significant reduction in the incidence of adverse outcomes in fetuses who underwent nephroamniotic shunting, compared with patients who did not receive treatment.In 4 cases, vesicoamniotic shunting for PUV disorder was performed (these cases were not included in the study). It was noted that in some cases, the renal pelvis was not completely emptied during this type of shunting. This phenomenon can be explained by a combination of upper and lower urinary tract obstruction. In such cases, it is rational to perform bilateral nephroamniotic shunting. Another well-known study, performed in 2009, published the results of prenatal interventions for severe hydronephrosis with oligohydramnios in the second trimester.15 Only 8 (57%) of 14 patients with PUV disorder were born alive. Five (63%) of the eight children born alive had a glomerular filtration rate (GFR) of less than 70 ml/min/1.73 m2 with an average follow-up period of 11.6 years. It should be noted that all patients in this cohort had favourable intrauterine urine indicators, but this did not predict changes in renal function in the neonatal period.15 We took into account the data obtained by our colleagues, and one of the indications for nephroamniotic shunting was the presence of blood flow in the kidney parenchyma. According to our data, the sensitivity and specificity of the power Doppler for evaluating the function of the renal parenchyma before shunting surgery were 87% and 92%, respectively.
S.Wu and M. Johnson presented a study on the specifics of prenatal diagnosis and evaluation of the results of intrauterine treatment for lower urinary tract obstruction. In experimental models, the authors found a relationship between urinary tract obstruction and the development of fetal fibrocystic kidney dysplasia, which indicated irreversible changes in the renal parenchyma. These researchers demonstrated that shunting improved survival, but neonatal morbidity remained a severe problem.16,17 The data of our study determined a significant difference in morbidity between patients in the main group with nephroamniotic shunting (7.6 %) and the control group (21.4%) [p<0.05]. It is evident that high hopes can be associated with the introduction of bilateral nephroamniotic shunting, which will significantly improve postnatal results in the future. In 2005, Biard J. M., Johnson M. P., Carr M. C., Wilson R. D., and Hedrick H. L. reported a survival rate of 91% of newborns up to 1 year of life after vesicoamniotic shunting surgery. The authors noted that patients with PUV disorder tended to have more favourable outcomes than patients with urethral atresia or "Prune Belly Syndrome".18 In our practice, PUV disorder was observed in all cases of infravesical obstruction.
Thus, to date, the question of the use of shunting operations in fetuses with obstructive malformations of the urinary system is controversial. There are still ongoing discussions concerning the pathogenesis of urinary tract obstruction and the mechanisms of its effects on fetal nephrogenesis. Obviously, the more information that is published about the results of prenatal interventions, the better we will know which patients need these interventions. Based on the data obtained regarding the improvement of renal function, we will continue our research on the application of nephroamniotic shunting and expand the indications for its use.
None.
None.

©2021 Kosovtsova, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.