Journal of
eISSN: 2373-4426


Case Report Volume 13 Issue 3
1Neonatologist and Pediatrician, Instituto Nacional de Perinatología, Mexico
2Neonatologist and Pediatrician, Hospital Ángeles Lomas, Mexico
3Pediatrician, Centro Médico ABC Campus Santa Fe, Mexico
Correspondence: Alejandra Prian Gaudiano, MD Pediatrician, Centro Médico ABC Campus Santa Fe, Vasco de Quiroga 4299 CP 05370 Ciudad de México, Mexico, Tel 5585349245
Received: October 27, 2023 | Published: November 10, 2023
Citation: González-Gordillo C, Orozco-Soto LE, Sarmiento-Aguilar A, et al. Newborn from the third deed with severe Transient Neonatal Myasthenia Gravis: Case report. J Pediatr Neonatal Care. 2023;13(3):225-227. DOI: 10.15406/jpnc.2023.13.00521
Case report: Hereby we present a case study of a newborn with severe Transient Neonatal Myasthenia Gravis (TNMG). He had a maternal history of Myasthenia Gravis and the pregnancy was urgently terminated due to loss of fetal well-being. After birth, the newborn presented generalized hypotonia and apnea that required orotracheal intubation. Due to the maternal history and clinical picture, a diagnostic-therapeutic test was performed with intramuscular neostigmine with a transient improvement in respiratory effort. Intravenous immunoglobulin and pyridostigmine were administered, gradually presenting improvement in muscle tone until weaning from mechanical ventilation. Finally, he was discharged to intermediate therapy where he received rehabilitation.
Conclusion: Babies of pregnant women with Myasthenia Gravis should be considered at risk of developing TNMG. Management should be directed to support vital functions. With prompt diagnosis and proper management, most newborns will experience a full recovery. The present case was approved by the ethics review board and consent was obtained.
Keywords: transient neonatal myasthenia gravis, congenital myasthenia gravis, neostigmine, pyridostigmine
Myasthenia gravis (MG) is a disease that affects the neuromuscular junction causing muscular weakness.1,2 In the neonatal period we can identify two types: congenital MG, caused by a genetic defect, and Transient Neonatal Myasthenia Gravis (TNMG),3 that presents in 10-20% of the newborns of a mother with MG.1 The reason why it doesn´t present in all the newborns of a mother with MG is not well known, there is no way to predict if a newborn will be affected by the disease, and the disease itself is not related with the severity of the mother´s disease or her antibody titers.4 The only association that has been found according to some studies is a protective effect of thymectomy in the mother, on the risk for a newborn to develop TNMG.5
During pregnancy, antibodies against muscle specific kinase (anti-MuSK) and against the acetylcholine receptor (anti-AChR)4 pass through the placenta, causing the blockage of the neuromuscular junction in the newborn.6 In these cases, TNMG presents after birth and resolves spontaneously at about 2 months of age.4 4The most frequent clinical presentation is hypotonia and weakness in sucking.6
Here, we report a severe case of a newborn with TNMG, born from a mother with MG that had received pharmacological treatment prenatally. Symptoms in this case presented since the moment he was born, needing mechanical ventilation and intensive pharmacological treatment.
The patient was a term newborn, born from the third deed of a 35 year old mother (history of 1 miscarriage and 1 cesarean section of an alive newborn who also had TNMG). She was diagnosed with MG 6 years before this case, with anti- acetylcholine titers of 5.5 nmol/L. During pregnancy she was treated with 60 mg of pyridostigmine every 4 hours, 100 mg of azathioprine and 30 mg of deflazacort every 24 hours. She had an adequate prenatal medical care since the first trimester of pregnancy. The maternal-fetal ultrasound of the second trimester was reported without any structural abnormality and without risk of chromosomopathies.
The mother came into the tocosurgical unit because of fetal tachycardia detection, and the interruption of pregnancy by c-section was indicated. A male newborn was obtained without muscle tone. He required orotracheal intubation during neonatal resuscitation because of apnea that didn´t resolve with positive pressure ventilation. He obtained an Apgar score of 3/7 and was admitted to the Neonatal Intensive Care Unit (NICU) with the suspicion of TNMG. At the physical examination, he was hypoactive, had generalized hypotonia, no spontaneous respiratory effort, muscle strength in the four extremities of 2/5 and hypotrophy of the shoulder girdle. He had positive deep tendon reflexes, no clonus. Thorax x-ray revealed a bell chest image (Figure 1).
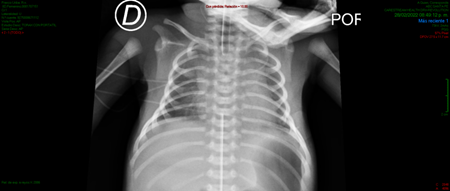
Figure 1 Thorax X-Ray revealing a bell-chest image, in a patient with Transient Neonatal Myasthienia Gravis (TNMG).
He was evaluated by a pediatric neurologist, who made a diagnostic-therapeutic test with intramuscular neostigmine (0.04 mg/kg/do), after which he presented a cholinergic reaction with bradycardia, and the drug was suspended. Even when the dose was not completed, the test was considered positive because the respiratory effort transiently improved.
We measured anti-acethylcoline antibodies and found them positive. When he was 24 hours old we initiated intravenous immunoglobulin, completing a total dose of 2 g/kg/do administered within 5 days, obtaining a partial improvement of symptoms.
We initiated treatment with pyridostigmine when he was 6 days old, increasing the dose until 7 mg/kg/day where we achieved a good clinical response and were able to wean the mechanical ventilation. After that, the patient received oral, suction and swallowing rehabilitation and was finally discharged.
Myasthenia gravis is an autoimmune disease where antibodies attack the postsynaptic membrane of the neuromuscular junction causing muscular weakness and progressive fatigue.2,3 In women, the incidence has a maximum peak between the second and third decades of life, which matches the fertility period.5
Affection to the newborn occurs vertically through the transplacental pass of antibodies. The mechanisms by which receptors are affected are: acceleration of receptor degradation, complement activation and amplification, promoting in this way destruction of the postsynaptic membrane and impeding acethylcoline reaching the receptors.2 In the study made by Eymard et al., they report that MG severity and treatment in the mother, doesn´t correlate with the newborn clinical condition.7 Nevertheless, they found a narrow correlation between maternal antibody titers and the onset and severity of the newborn´s disease. Among the 15 mothers with high antibody titers (more than 60 nmol/L), 13 had an affected newborn, 6 of them with a severe disease. On the other hand, all the mothers with low antibody titers (less than 10 nmol/L) had an asymptomatic baby. Therefore, they concluded anti-AChR titers are predictive of TNMG.7
Between the weeks 17 and 22 of gestation the serum concentration of IgG in the fetus is of only 5-10% of the mother’s concentration. At birth, both concentrations are similar.8 Even when AChR antibodies are found in almost all the newborns of a mother with MG, their pathogenic role is unclear because only some babies are symptomatic, which is why measuring antibody titers in all newborns from a mother with MG is not usually suggested.9
Usually, a compatible clinical scenario in the context of a mother with MG is enough to make the diagnosis, which is why a detailed perinatal clinical history should always be made,9 including the number of pregnancies, because it has been suggested that risk could increase accordingly.8 This has been confirmed in the presented case, because the patient had an affected previous brother who didn´t have such a severe disease. Finally, positive antibody titers can confirm the diagnosis.9
Every newborn of a mother with MG should be observed at the hospital for 3 days, because babies born from mothers who were treated with anticholinesterasic agents can show mild or no symptoms in the first 24 hours of life, but will always show them before 72 hours in case they are affected.10 Respiratory insufficiency, broncoaspiration and pneumonia are rare complications that make it necessary to observe these patients in the NICU.8
There have been two clinical forms of TNMG: typical (71%) and atypical (29%). Clinical characteristics of the atypical form include the presence of congenital multiple arthrogryposis in the fetus or newborn. The severity is variable and doesn´t correlate with the number of pregnancy or severity of mother´s illness.11 In the typical form of TNMG, the usual clinical findings include poor suction and generalized hypotonia. Other clinical manifestations are a weak cry (60-70%), diplegia or facial paralysis (37-60%), difficulties to swallow or suction (50-71%) and respiratory distress. Ptosis (15%) and ophtalmoparesis (8%) are less frequent. Respiratory distress that requires mechanically assisted ventilation may occur in severe cases (29%),11 as it occurred in ours.
Once we´ve had the first clinical signs we need to administer an anticholinesterasic agent to make the diagnosis. A positive response is defined as the transitory correction of a neurological unequivocal deficit, as difficulty to suction or swallow. The most common pharmacological test is to apply intramuscular or subcutaneous neostigmine in an only dose of 0.04 mg/kg.10
Treatment should be focused in supporting vital functions, especially ventilation and an adequate nutritional status until weakness gets better.10 As IgG antibodies from the mother disappear gradually from the newborn´s bloodstream, muscular weakness improves until recovering a normal function. Weakness usually lasts 4 weeks, but is worse during the first one.8
In around 20% of the patients, symptoms are mild and treatment consists in small but frequent feedings and continued medical observation. The other 80% of the patients have a moderate to severe disease presentation.10 When this occurs, anticholinesterasic agents are the first line drugs, the most common, neostigmine, at a dose of 0.04 mg/kg every 4 to 6 hours. Nevertheless, in the case of our patient, he presented bradycardia with this drug, which is why we used piridostigmine, which is an alternative with less muscarinic secondary effects.9
The dose of anticholinesterasic agents can be gradually increased until suction, swallowing and respiratory distress gets better. A gradual weaning of the drug is recommended when symptoms have improved and antibodies are negative.10
Only rare cases need to be treated with intravenous immunoglobulin (IGIV) or plasmapheresis.8 Our patient was one example, who needed a total 2 g/kg/day of IGIV during the first days of life.10
With a correct diagnosis and prompt treatment, most newborns with this condition will fully recover, generally before 2 months old (90%) or before 4 months old (10%). After recovery, they don´t have a greater risk of developing MG later in life and usually don´t have any secuelae.10
In conclusion, babies born from mothers with MG are always at risk of developing TNMG, which can be a fatal disease if it is not diagnosed and treated promptly. A compatible clinical scenario in the context of a mother with MG should be enough to make the diagnosis, give a correct medical treatment on time and achieve spontaneous remission.
None.
None.
The authors declare that there are no conflicts of interest.

©2023 González-Gordillo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.