Journal of
eISSN: 2373-4426


Case Report Volume 2 Issue 6
University of Puerto Rico Medical Sciences Campus, Puerto Rico
Correspondence: Ortiz Justiniano V, Puerto Rico Childrens Hospital Ofic. 302 Carr Num. 2, Km 11.7 Edif. Medical Plaza Bayamon, PR 00960, Puerto Rico, Tel 787-474-5423, Fax 787-523-2768
Received: June 11, 2015 | Published: September 8, 2015
Citation: Quintero H, Justiniano OV, Vazquez Z, Rivera A (2015) Management of Omphalocele with Intestinal Perforation: A Case Report. J Pediatr Neonatal Care 2(6): 00099. DOI: 10.15406/jpnc.2015.02.00099
Congenital abdominal wall defects present a huge challenge for pediatric surgeons in the care of neonates. The risks of infection and restriction of blood supply to abdominal organs challenge the surgeons’ capacity to restore the stability of the patient. Omphalocele is a defect of the abdominal wall where the organs protrude enclosed within a membranous sac. Carrying a mortality rate of 34%, an incidence of 1 in 4,000 live births and being more common in males than in females1,2 makes it more difficult for the surgeon to manage complications. There are few reports of intestinal perforation in patients with omphalocele.3 We report the case of a 2‐days‐old boy who presented with omphalocele that required surgical excision of the membranous sac for management of intestinal perforation as a life saving procedure.
Keywords: congenital abdominal wall defect, omphalocele, intestinal perforation, neonate, spring‐loaded silo
WGA, weeks of gestational age; NICU, neonatal intensive care unit; NGT, French nasogastric Tube; FFP, fresh frozen plasma; PRBC, packed red blood cells; POD, post-operative day; OR, operating room; VSD, ventricular septal defect
Omphalocele presents an incidence of 1/4000 live births and 10‐30 % associated chromosomal anomalies. It is more common in pregnant women over 30 years of age. It has been reported that 10 to 18 % of the cases present with rupture of the membranous sac in uterus and 4 % present at childbirth. Patients with intact membranous sac are approximately 48 % of the cases.1 Omphalocele is a protrusion of the viscera from the umbilical ring in the midline region of variable sizes, covered by a membranous sac, which is composed of two layers. The internal surface is composed of peritoneum and the external surface of amnion, with Wharton’s jelly between the layers.2 Gastroschisis, on the other hand, is a full thickness defect to the right of the umbilical cord. Omphalocele can be repaired through primary closure or staged procedures. In primary closure, the membranous sac is incised to divide and ligate the umbilical vessels. Then, the abdominal wall musculature is manually stretched by placing a finger into the peritoneal cavity in each quadrant around the defect. This is followed by fascial closure with interrupted sutures.4 Tight fascial closure might cause the complication of abdominal compartment syndrome. In patients that do not tolerate primary closure or skin flap closure, a staged procedure must be considered, such as the use of a biodegradable mesh. A simple method consists of placing a prosthetic spring‐loaded silo to allow slow reduction of the viscera, as used for gastroschisis. These are commercially available or can be constructed by the surgeon. These methods are contraindicated if there is an intestinal perforation. Giant omphalocele (>5 cm diameter) is mostly considered another entity due to the challenge it poses in management. The most common technique used nowadays is daily application of silver sulfadiazine covered by cotton free gauze and elastic bandage. We are presenting an alternative management in a patient with omphalocele that required artificial rupture of the membranous sac to treat intestinal perforation and peritonitis.
Case of a 2‐days‐old boy delivered at 36 weeks of gestational age (WGA) in a referring institution, by a 36 years old primigravid via spontaneous vaginal delivery after spontaneous rupture of membranes. Pregnancy was complicated with gestational diabetes and with maternal Group B Streptococcus infection, both adequately treated. The birth weight was 2.3 kg and APGAR scores were 8 and 9, at one and five minutes, respectively. The baby was transferred to the neonatal intensive care unit (NICU) for close monitoring and antibiotic therapy due to the finding of omphalocele, which was not diagnosed in the antenatal period. The patient was transferred to our facility at two days of age due to worsening abdominal distension and acidosis. Upon admission at our intensive care unit, he was found critically ill and on assisted ventilation. On physical exam he had abdominal distension, increased collateral circulation and the presence of an omphalocele of 5 cm with air bubbles seen inside the membranous sac Figure 1. Laboratory studies showed severe metabolic acidosis with concomitant respiratory acidosis with a pH of 7.18, pCO2 of 36 mmHg, and bicarbonate levels of 13.5mEq/L. Hemoglobin was 8.5 g/dL, there was mild thrombocytopenia, prolonged coagulation profile and hyperglycemia. The patient was kept on nothing by mouth, and an 8 French nasogastric tube (NGT) was placed for bowel decompression. Also, a bladder catheter was in place. Treatment with intravenous antibiotics piperacillin/tazobactam and gentamycin was started. Fluid challenges were administered at 20 ml/kg and fresh frozen plasma (FFP), as well as packed red blood cells (PRBC) were transfused simultaneously.
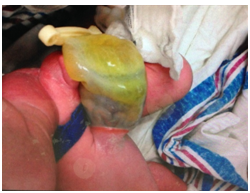
Figure 1 Physical findings of patient upon arrival to the intensive care unit. Air bubbles can be seen inside the membranous sac.
Surgical excision of the membranous sac, extension of umbilical ring and exploration of bowel was performed. Upon examination of the bowel, a distal ileal perforation with areas of ischemia was found with gross cavity contamination Figure 2. As a salvage measure, the decision was made to perform an ileal resection, clip the distal ileal segment and mature an end Brooke ileostomy to the left side of the abdominal cavity through a separate incision Figure 3. During the procedure, the baby developed hypotension and hypoperfusion, requiring multiple fluid challenges plus inotropic administration. The patient remained unstable, and the bowel was left covered with Vaseline gauzes and wrapped with gauzes to allow daily assessment of bowel viability until his condition was optimum for silo placement. Parenteral nutrition was started. On post-operative day (POD) #3, patient was taken to the operating room (OR) for placement of a 5 cm spring‐loaded silo. Serial reductions were performed bedside and evaluation of ostomy function was adequate. During his stay, pediatric cardiology services were consulted to assess for cardiac anomalies. Patient was found to have a ventricular septal defect (VSD) that eventually resolved upon discharge. Moreover, bilateral hydronephrosis was found on a screening renal ultrasound. Upon evaluation of renal function, no abnormalities were found. The nephrologist recommended a follow-up study at four months of age to reassess renal function. By POD#12, primary closure of open wound was performed with normal intravesical pressures in the postoperative period.
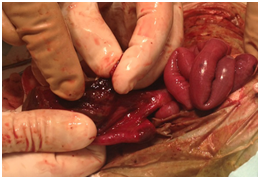
Figure 2 After surgical excision of membranous sac, a distal ileal perforation with surrounding areas of ischemia was found.
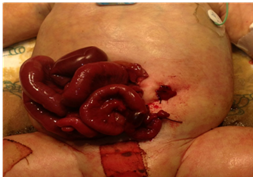
Figure 3 Ileal resection was performed with an end Brooke ileostomy to the left of the abdominal cavity.
His course was complicated by intestinal obstruction due to stomal stricture that required ostomy revision on POD#17. Subsequently, feedings where re-started with adequate return of bowel function. Also, his course was altered by wound dehiscence requiring bedside local care, fungemia by Candida tropicalis successfully treated with fluconazole, bacteremia with multidrug resistant Acinetobacter baumanii treated with polymixin B, and ileostomy prolapse without obstruction. After infectious processes resolved, he was taken to the OR on POD# 48 for ileostomy closure, enterolysis, anastomosis and primary closure with wound revision. He progressed successfully and was discharged on POD# 57 showing adequate wound healing. Patient was tolerating formula feedings and stooling adequately for age.
Omphalocele was described in the sixteenth century printed works of Ambroise Paré. The first successful repair of omphalocele was reported by Hey in 1802.5 This entity poses quite a challenge to manage, as various options exist depending on the presentation and possible associated findings. The most common associated findings are congenital anomalies, mainly cardiac defects, such as ventricular septal defect, atrial septal defect, ectopiacordis, tricuspid atresia, coarctation of the aorta and persistent pulmonary hypertension. Other associated conditions are gastrointestinal (gastroesophageal reflux), neurological (neural tube defects), as well as genitourinary and chromosomal abnormalities. Intestinal perforation is uncommon in omphalocele, with few cases reported in the literature.3 Also, there have been reports of bowel injury, namely strangulation, in ruptured omphalocele sacs with associated midgut volvulus.6
The most probable etiology that can explain what occurred in our patient is bowel impingement by to the omphalocele ring, leading to bowel ischemia and perforation at its most distal segment. Some may consider that vaginal delivery might have had an impact in the pathophysiology of the disease, yet the presence of abdominal wall defects is not an obstetrical emergency. Recent studies have shown that there is no difference between cesarean section and spontaneous vaginal delivery in the prevention of injury to the bowel or tearing of the omphalocele sac. The mode of delivery is determined based on obstetrical indications and not on the presence of an abdominal wall defect.7 In our case, the diagnosis of omphalocele had not been made in the antenatal period, thus labor was induced after spontaneous membrane rupture for vaginal delivery not causing any injury to the membranous sac (the membranous sac was intact at birth).
Since our patient presented with hemodynamic instability, a life saving procedure was performed as described. The treatment of choice for small omphaloceles (<5cm diameter) is primary closure, which includes all layers, except the skin. However, in our case the neonate presented a distal ileal perforation Figure 2 with cavity contamination, for which the membranous sac was purposely excised. An end ileostomy was created to the left of the abdominal cavity after resection of the ischemic/ruptured ileal segment as a salvage measure. This presented a challenge for management because the membrane had been excised. We were then committed to perform a staged repair by placing a silo, as in the management of gastroschisis, due to the presence of peritonitis, and hemodynamic instability secondary to sepsis.
In our case there was presence of infection and bowel perforation. The membranous sac had to be excised in order to deal with this complication. We are aware of other methods available for the repair of omphalocele and/or giant omphalocele, including skin flap closure, tissue expander or mesh placement, external compression with bandages or pneumatic devices, and non‐operative initial treatment. A non‐surgical treatment approach consists of applying an antiseptic solution to an intact membrane sac that induces epithelialization. This could result in a ventral hernia, which can be subsequently repaired. It has been reported in the literature that initial topical coverage with silver sulfadiazine is associated with excellent outcomes for infants with giant omphaloceles who cannot undergo immediate closure.8 These surgical methods could not be used in our case due to the presented complication. Moreover, the membranous sac had to be excised, which prevented us to follow a non‐surgical management.
There are diverse strategies available for closure of abdominal wall defects. Nonetheless, our patient presented a rare complication among patients with omphalocele defect. We implemented an approach, vastly described and widely accepted for management of gastroschisis, in a patient that originally presented with omphalocele, obtaining good outcomes. This method does not constitute a standard of care treatment in omphalocele, but certainly is an excellent option available in the surgeon’s armamentarium when managing a similar patient.
None.
The authors declare that there are no conflicts of interest.
None.

©2015 Quintero, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.