Journal of
eISSN: 2373-4426


Case Report Volume 13 Issue 3
1Department of Pediatrics, University Santiago de Compostela (USC), Spain
2Department of Pediatric Surgery, University Santiago de Compostela (USC), Spain
3Department of Radiology, University Santiago de Compostela (USC), Spain
4Department of Thoracic Surgery, University Santiago de Compostela (USC), Spain
Correspondence: Pilar Fernández Eire, Department of Pediatrics, Pediatric Surgery Hospital Alvaro Cunqueiro Estrada Clara Campoamor- 325, 36212 Vigo, Spain, Tel 34-986811111
Received: October 21, 2023 | Published: November 13, 2023
Citation: Carballo LB, Eire PF, Mañas Uxó JJ, et al. Is it necessary to monitor any degree of hemoptysis in childhood?. J Pediatr Neonatal Care. 2023;13(3):229-231. DOI: 10.15406/jpnc.2023.13.00522
Inflammatory myofibroblastic tumor of the lung is a very rare tumor in childhood. We present the clinical case of an 11-year-old boy who came to the emergency room due to very mild hemoptysis that lasted a few hours, without other symptoms or signs associated. The rapid onset of hypovolemic shock forced us to perform an urgent thoracotomy. A large tumor with uncontrollable bleeding was found and a right pneumonectomy was performed.
Keywords: inflammatory myofibroblastic tumor, lung, children, hemothorax
Inflammatory myofibroblastic tumor (IMT) or inflammatory pseudotumor is the rarest lung neoplasm in the paediatric age group and accounts for 0.04-1% of primary lung tumors.1,2 Lung location is the most frequent in pediatric age, although it can be located in other thoracic, abdominal organs (mainly in the mesentery, liver, bladder, stomach) and even in the central nervous system and heart.2,3
The WHO classification of soft tissue and bone tumors classified IMT as an intermediate tumor and defines it as a lesion composed of a proliferation of myofibroblastic spindle and stellate cells with abundant eosinophilic cytoplasm mixed with infiltrative plasma, inflammatory cells, eosinophils and lymphocytes.3–5
The characteristic of the tumor, common to any location, is anaemia. In 50% of cases it is silent, being diagnosed incidentally in another examination. Symptoms usually include cough, weight loss, fatigue and hemoptysis, but the clinical spectrum is very broad, from an asymptomatic patient who is accidentally diagnosed in a radiological test requested for another reason to severe shock due to massive hemothorax.1–6
Its etiology is uncertain but some authors speak of an exaggerated response to some type of stimulus as surgery, infection, steroid hormone use, an autoimmune response and abnormal expresion and genetic mutation of the anaplastic lymphoma kinase (ALK) gene.7
It requires differential diagnosis with other lung tumors such as neuroblastoma, pleuropulmonary blastoma, hemangioma, pulmonary sequestration, lymphoma, immature teratoma and metastases from other tumors, which are the most frequent and the easiest to differentiate due to their size and number.1
The diagnosis is basically anatomopathological, characterised by a localised proliferation of monoclonal inflammatory cells, usually plasma cells, lymphocytes, eosinophils, spindle- shaped mesenchymal cells and myofibroblasts.3 ALK expression, detected in half of patients with IMT, has been reported to be associated with better prognosis.7
The treatment of choice is a surgical with complete resection with negative margins, which is necessary, ideally, during early stages of tumor growth, although sometimes this is not possible due to invasion of vital structures. Lung surgery includes a sementectomy, lobectomy or even a pneumonectomy when the bleeding is uncontrollable.3
Preoperative embolisation can be very useful in order to reduce intraoperative bleeding.5,9 In cases of incomplete surgery or advanced disease with involvement of vital structures, chemotherapy is an option which could be used.8,10
An 11-year-old boy, with no relevant history, went to the pediatric emergency room due to mild hemoptysis that lasted 4 hours. He had no digestive or respiratory symptoms or signs that could justify the appearance of blood in his mouth.
The child was in good general condition. Pulmonary auscultation showed hypoventilation of the right hemithorax, so a chest x-ray was performed, which revealed an increase in density in the lower 2/3 of the right hemithorax and displacement of the mediastinum to the contralateral side (Figure 1).
Laboratory tests showed hemoglobin of 10.6 g/L. There was hypoalbuminemia, hypophosphatemia and hypomagnesemia as the only biochemical alterations. The rest of the parameters were normal, including blood gas and coagulation study.
The thoracic scan showed a large tumor measuring 17 x 13 x 11 cm that caused an almost complete collapse of the right main pulmonary artery and deviation of the mediastinum to the contralateral side (Figure 2). The study was completed with a chest MRI, limited by the patient's instability. Comparing it with the previous scan, a greater content of active bleeding was observed at the level of the right lower lobe (Figure 3A, B).
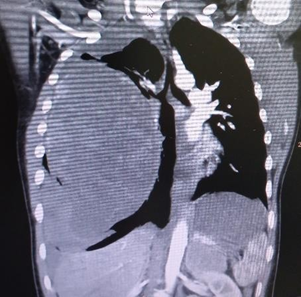
Figure 2 Chest CT Scan: mass measuring 17 x 13 x 11 cm with a significant collapse of the main pulmonary artery and displacement of mediastinal structures.
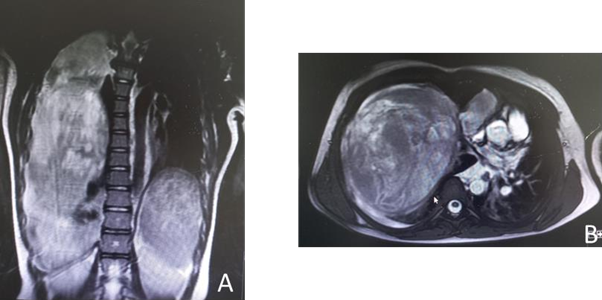
Figure 3
A. MRI findings show that the mass apparently has intrapulmonary characteristics.
B. The anterior and inferior chest wall contacts the mass without being able to rule out wall involvement.
A few hours later, the patient suffered rapid clinical and hemodynamic deterioration, developing hypovolemic shock that required 3 transfusions of concentrated red blood cells, fresh plasma, vitamin K and support with inotropic drugs. The interventional radiologist ruled out the possibility of embolization and due to the impossibility of further conservative treatment, an urgent thoracotomy was decided. A median sternotomy and a right pneumonectomy were performed, including the tumor mass. When the chest was opened, massive bleeding occurred with serious hemodynamic compromise that required 6 concentrated red blood cells.
The subsequent evolution was satisfactory. The patient was extubated after 48 hours, with respiratory support progressively withdrawn. After 7 days he was discharged.
Pathological anatomy confirmed the total removal of an inflammatory myofibroblastic tumor.
With this clinical case we have learned that any degree of hemoptysis must be investigated, no matter how mild it may seem.
None.
All the authors approved the final versión of this paper.
The authors have not declared a grant for this research from any funding agency in the public, comercial or not-for-profit sectors. This investigation has not received specific contributions from public sector, comercial sector or nonprofit entities.
The authors declare that there are no conflicts of interest.

©2023 Carballo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.